The effect of a non-ionic surfactant on particles removal in post-CMP cleaning was investigated. Bychanging the concentration of the non-ionic surfactant, a series of experiments were performed on the 12 inch Cupattern wafers in order to determine the best cleaning results. Then the effect of the surfactant on the reduction ofdefects and the removal of particles was discussed in this paper. What is more, the negative effect of a non-ionicsurfactant was also discussed. Based on the experiment results, it is concluded that the non-ionic surfactant couldcause good and ill effects at different concentrations in the post-CMP cleaning process. This understanding willserve as a guide to how much surfactant should be added in order to achieve excellent cleaning performance.
Owing to its unique electrical properties such as low resistivity and high resistance to electromigration, copper (Cu) hastaken the place of aluminum (Al) and become the main material for interconnection. However, it is difficult for copper toassociate with reactive ion etching (RIE), which is usually usedin aluminum interconnection. To solve the problem, a Damascene structure is implemented to realize the copper on-chip interconnects. During the Damascene process, chemical mechanical polishing (CMP) is utilized to remove the overburdenedcopper, Ta/TaN barrier and so on, gaining an excellent surfaceplanarity. Unfortunately, this process leaves a large amount ofcontamination on the wafer surface. The major contaminantsinclude BTA, abrasive from the slurry, undesired metallic ionsand other chemical components, all of which must be eliminated in the post-CMP cleaning process.
Colloidal silica based slurry collocating with H2O2 is currently used in the Cu CMP process, due to its excellent abilityto remove the tantalum layer; however, it also shows a strongtendency to adsorb colloidal silica on the copper surface, whichaccounts for most of the particles residual on the wafers polished. Moreover, the chemical components of slurry, thefragments of PVA brush and so on may also contribute to theresidual particles. Those particles mentioned, if not removedcompletely, may cause a short circuit or open circuit after transferring the desired circuit, which eventually seriously influencethe performance of the device or even result in its failure.
Many efforts have been devoted to the removal of particles, especially the colloidal silica abrasives from the polishedcopper surface. A surfactant is commonly used in postCMP cleaning, since it can significantly reduce the surface tension of the liquid and interfacial tension, which is favorable tothe particles removal. So a non-ionic surfactant is chosen as itcannot ionize in water and does not cause ion contamination.Experiments improved the adsorption state of particles, whichcan be controlled and changed with a non-ionic surfactant.
In this study, a novel alkaline cleaning solution, namelyFA/O cleaner, was developed for post-CMP cleaning. In orderto enhance the efficiency of removing particles, the FA/O nonionic surfactant, a kind of complex surfactant, was added in thecleaner. Therefore, the effect of the FA/O non-ionic surfactantwas investigated in this paper.
The CMP process was carried out on an Applied Materials300 mm Reflexion LK tool, which was designed for a threestep copper CMP process and the requirement of dry in anddry out. Therefore, a three-step polishing scheme was used:step one was to remove bulk copper and gain an initially planarized surface; step two was to remove the residual copperand stop on barrier layers; and step three was to clean barriermetal and some of the dielectric. All the wafers were polishedwith the same Cu and barrier slurries. The slurry based on colloidal silica (14 wt%) with benzotriazole (BTA) and hydrogenperoxide (H2O2/ was used in step three for barrier CMP. Subsequently, the post-CMP cleaning process was performed onthe same machine. Firstly, the polished wafers were washedwith megasonics. Then the wafers were cleaned in PVA brushbox 1 and box 2. Finally, the wafers were blow-dried in an IPAvapor dryer. Once the whole process was completed, the 2825broadband optical pattern wafer defect inspection tools (KLATencor) and SEMVisionTM G4 defect analysis platform (Applied Materials) were used to determine the cleaning performance. All the experiments were performed utilizing 12 inchcopper pattern wafers. Figure 1(a) shows the schematic of pattern copper wafers, and Figure 1(b) shows the model of someresidues on the wafer surface after the CMP process.
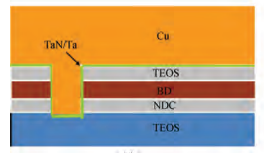
Fig1
As for the physically adsorbed colloidal silica abrasives,the osmotic agent molecule that the surfactant contains can permeate and expand between the wafer and the colloidal silicaabrasives, as shown in Figure 5(b). The dispersant moleculeholds up the colloidal silica particles and replaces it on thewafer surface. At the same time, the silica is surrounded bya layer of complex surfactant molecules, as shown in Figures5(a) and 5(b). Once desorbed from the wafer surface, the colloidal silica abrasives are easily removed by the PVA brush andwashed away with the flow fiuid.
下一篇: 用于外延应用的原位低温硅表面清洁的氯蚀刻