There is considerable recent interest in Cu,ZnSnS. (CZTS) dueto its excellent properties for use in solar cell applications.!,2 It islow-cost material formed from relatively nontoxic and abundantelements and in addition has both a useful bandgap of l.5 eVand a high absorption coefficient (>10' cm-').3 This p-typesemiconductor can be viewed as a derivative of the chalcopyritesCulnS, or CuGaS) in which, the less abundant elements, In orGa, are replaced with more available Zn and Sn.4,s A photoharvesting efficiency for a cell derived from this material of 9.6has recently been reported.6
The methods that have been used for the preparation of CZTSthin films include: Rf sputtering,’ co-evaporation, hybrid sputtering’ sulfurization of electrochemically deposited metaprecursors2,o photochemical deposition1 sol-gel sulfuriza-tion,12 sol gel spin coated deposition13 pulsed laser depositionland spray pyrolysis.is In addition, nanoparticulates of CZTShave been prepared by solution methods.1 However, many ofthese methods have various potential short-comings, such ascontamination with the binary sulfides (various phases of CuSCuS, ZnS or SnS) or a lack of stoichiometry control.17 Hence newmethods to prepare high quality thin films of CzTS are needed.
CVD is a well known method for the preparation andproduction of high quality compound semiconductor thinfilms.is To the best of our knowledge a CVD route to CZTS hasnot been reported. This omission is due to the difficulties offinding suitable precursors for this quaternary system. Indepositing homogenous alloys, the decomposition rates of theindividual precursors delivering the elements need to be matchecand there should not be adverse interactions between theprecursors.19 This approach has been successfully developed inthe tailored synthesis of complex oxides such as lead zirconiumtitanate by choosing precursors for different component metalswith similar decomposition temperatures.20 By utilizing ourexperience with precursordevelopment for various CVD techniques, we identified a set of suitable precursors for thedeposition of CzTS thin films by aerosol assisted (AA) CVD.2Simple diethyldithiocarbamato complexes of copper [Cu(S,CNEt,)2] (1), zinc (Zn(S,CNEt)] (2) and the alkyl derivativeof tin (Sn(Bu)2(S,CNEt)] (3) were used in combination todeposit phase pure material.
Differential thermogravimetry (DTG) analysis of thecomplexes (1),(2) and (3) show that all three complexesdecompose at similar temperatures in DTG at 284 °C for (1).303 °C for (2) and 300 °C for (3) to their corresponding metalsulfide. A mixture of three complexes should hence be suitablefor the deposition of quaternary Cu,ZnSnS, films by AACVD.In a typical AACVD experiment a 2 : 1 : I molar ratio of Cu, Znand Sn complexes were dissolved in 10 mL of toluene and thedeposition was carried out at 360, 400, 440 and 480 C with theargon flow rate of 160 sccm for 90 min. The deposited films at alltemperatures were specular, dark brownish, well adhered to theglass substrate and uniform over the entire substrate size of2 cm'.
The powder X-ray diffraction of the as deposited films fromexperiments at 360 and 400 C are shown in Fig. 1. The materialcan be identified as tetragonal CuZnSnS. (ICDD: 26 0575 or002 0672) stannite or kesterite. The diffraction peaks can be indexed to (112), (200), (220), (312),(008) and (332) reflections oftetragonal Cu,ZnSnS, with preferred orientation along the (1 12)plane. PXRD pattern also confirmed the absence of any binarysulfides such as CuS, CuS, SnS, or SnS2.
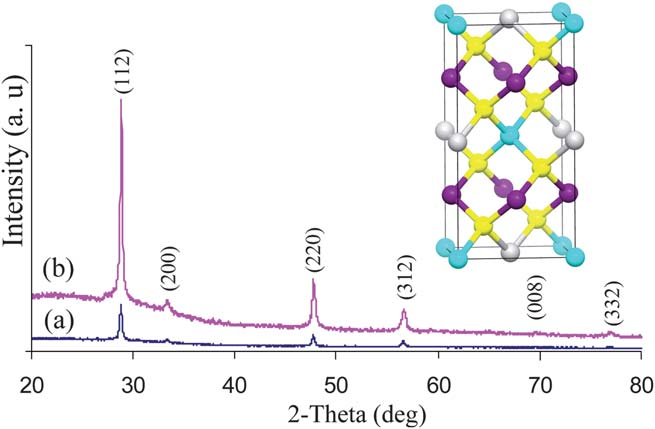
CuZnSnS4 can crystallize in both the kesterite (14) and stan-nite (14 2m) structures. The computed total energy value byFPLAPW method22 for kesterite phase is only 1.3 meV/atomlower in energy than for the corresponding stannite structure.23Since the lattice parameters and the structures of kesterite andstannite are very similar, both phases may exist in the samesystem and it is difficult to identify either of them separately bypowder X-ray diffraction data alone.
上一篇: 金属氧化物中有机污染物的影响
下一篇: 氧化对超薄氧化物的表面粗糙度的影响