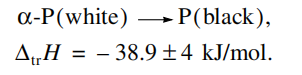INTRODUCTION
Indium phosphide is a Ill-V compound semiconductor widely used in optoelectronics.Experimental data for group MIA phosphides aredifficult to interpret because of the phosphorus polymorphism [1-6] (Table 1). Nine phosphorus allotropeshave been identified to date: two white (o, stable from0 to 195.4 K, and , stable from 195.4 to 317.3 K), fivered (P(I) to PV): P(I) and P(I) have not yet beenidentified with certainty), and two black (orthorhombicand amorphous) allotropes [71. Recently, Ruck et al.[8have described the structure of P(lV) red phosphorus(Hittorf’s phosphorus). According to their results, it hasa triclinic unit cell, in contrast to the monoclinic cellreported by Thurn and Krebs [9].
The lack of reliable data for different forms of phosphorus leads to misinterpretation of the enthalpy of formation of phosphides in studies where different allotropes of phosphorus are examined (in experiment orcalculation).For example, Sharifov and Gadzhiev [10showed by decomposing indium phosphide in a calorimetric bomb that the final products of that process wereindium and two forms of phosphorus: white and redThe white phosphorus (3345%) was separated fromthe red form by dissolving it in carbon disulfide, CS,The heat of transformation of red to white phosphoruswas not indicated in their report. A number of publications before 1988, including the handbook by Glushko4](Table 1), give inaccurate enthalpies of the transfor-mation