KOH etching is widely used in connection with micromachining of microelectromechanical systems ~ MEMS! . As an example, in the manufacturing of pressure sensors, an-isotropic KOH etching of silicon is used for making pressure sensitive membranes. Making a silicon membrane using KOH etching involves a six-step procedure. ~ i! Silicon nitride is deposited on a 4 in. silicon wafer. ~ ii! The silicon nitride is oxidized. ~ iii! Using photoresist in a standard photolithography technique, mask holes are defifined in the silicon oxide using a HF etching solution. (iv) The silicon nitride is now exposed to phosphoric acid at T 5 180°C where the unmasked areas are removed. In this process step, silicon oxide is not etched. (v) The silicon wafer with a silicon nitride mask is then exposed to a KOH etching solution, where unmasked silicon is removed. Membranes are formed when suffificient material has been etched away. This etching will also typically remove the silicon oxide mask. (vi) The nitride mask is fifinally removed in phosphoric acid. In the case of normal conditions the phosphoric acid will not etch the exposed silicon.
The motivation for this work was initiated by the discovery of iron oxide particles found on the silicon membrane and sidewalls of the cavity after such an anisotropic KOH etching of pressure sensors. Figure 1 shows such a particle contamination at 10,000 times magnifification and Fig. 2 shows at a higher magnifification a chain of these particles. Furthermore, it turned out that the KOH etching procedure did not deposit particles on either SiO2 or on Si3N4 masking layers, and when performing step ~ vi! in the procedure described above, the particles were not removed. Instead they caused pits in the silicon where particles were situated. In Fig. 3 a silicon membrane with particles and pits is shown. Both the presence of etch pits and particles is detrimental to sensors working in harsh environments since a coating with a passivating layer becomes diffificult if not impossible.1 Evidently the presence of iron particles is also detrimental to MEMS sensors since the iron will alter the behavior of the electronics that is present on the device.2
Experimental
Three experiments have been performed. Experiment I was designed to establish whether or not particle size and number grow with etching time. Experiment II was designed to give additional information on the technological aspect of wafer etching in the case of different dopant types and dopant levels, and experiment III was designed to investigate particle removal. In both experiment I and II the involved silicon~ 100! wafers were oxide stripped in a buffered HF etch for 30 s and subsequently rinsed in a deionized H2O rinsing solution ~ with a resistivity of 18 MV cm! with N2 bubbling for 3 min, prior to the KOH etching solution exposure. This was done in order to remove the native oxide from the silicon wafers, and thus ensure equal initial etching conditions. The KOH etching solution parameters are also common to experiment I and II, namely ~ i! the wafers are etched in an upright position in the etching solution, with the flflat pointing upwards. The flflat is a marker that is used to identify the @ 100# crystal direction of the single-crystalline silicon wafer. ~ ii! The KOH concentration is 29% (H2O:KOH 5 1.3:0.5 weight ratio! with a total solution of 9.6l, and the etching temperature is 80°C. At this KOH concentration and temperature the etch rate of the silicon is approximately 1.3 m m/min. ~ iii! The purity of the solution is limited by the purity of the KOH pellets supplied by the manufacturer. Maximum content of, for example, iron, cupper and nickel is in their respective order: 5, 1, and 1 ppm.
Characterization
In all the experiments the particles have been characterized in terms of morphology, quantity, and elemental composition with a LEO 1550 fifield emission gun SEM. In both experiment I and II the micrographs used for statistical evaluation were obtained at a magnifification of 10,000 times and with a zero tilt angle of the wafers in question. The numbers presented for experiment I are a result of 19 micrographs from each sample, obtained from across the sample with a 5 mm distance between each micrograph, beginning from the flflat and along an axis perpendicular to the flflat as shown in Fig. 4a. In experiment II, all SEM images have been obtained at 16 predefifined equidistant locations covering the whole wafer area as sketched in Fig. 4b.
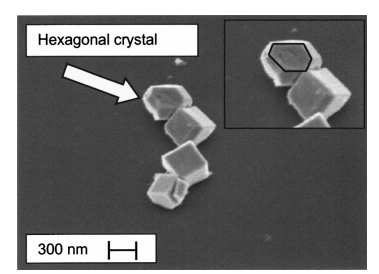
Fig1
Experiment II (the wafer-type experiment).—Like experiment I, microanalysis showed that selected particles contained Fe and O. Furthermore, the n-type wafers, the p-type and the p11 type wafers showed particle coverage similar to those described in experiment I. However, highly doped n-type wafers showed an extremely different behavior. In Fig. 9 a typical SEM image from an n11 wafer is presented. Spot testing using approximately 30 particle chains from Fig. 9 yields a size distribution as shown in Fig. 10. Evaluating Fig. 10 it is seen that the chain width and thereby the particle size is quite monodispersed. Similar spot testing was performed on all wafer types. In Fig. 11 the size distribution analysis performed on the p11 type wafer is presented. Evaluating Fig. 11 it is seen that the size distribution is less narrow than in the case of the n11 type wafer. However, it can be seen that there is a strong grouping around a particle size of 220 nm. As in experiment I the coverage was found on all obtained micrographs, and the mean value and standard deviation was derived for the four different wafer types. In Fig. 12, where the resulting numbers are presented, it is seen that the n11 type wafer has a coverage which is at least 16 times higher than any other wafer type and that the other wafer types have comparable coverage, although they are different in respect to dopant type and concentration.
Discussion
Iron source.—In both experiments, EDS shows that the particles contain Fe and O. A possible iron source is the KOH pellets used for making the KOH solution. The data sheet from the KOH manufacturer states that the Fe content in the pellets is below 5 ppm. Calculations show that if the particles are for, example, hematite Fe2O3 then at levels of only 5 ppm in the KOH pellets, there are 1000 times more Fe ions in the solution than on the wafers.
上一篇: 化合物半导体单晶片清洗技术
下一篇: 磷酸中二氧化硅的选择性湿法蚀刻方法