Abstract: We investigated the aluminum nitride etching process for MEMS resonators. The process is based on Cl2/BCl3/Ar gas chemistry in inductively coupled plasma system. The hard mask of SiO2 is used. The etching rate, selectivity, sidewall angle, bottom surface roughness and microtrench are studied as a function of the gas flow rate, bias power and chamber pressure. The relations among those parameters are reported and theoretical analyses are given. By optimizing the etching parameters, the bottom surface roughness of 1.98 nm and the sidewall angle of 83° were achieved. This etching process can meet the manufacturing requirements of aluminum nitride MEMS resonator.
Aluminum nitride (AlN) is an III-V group compound semiconductor material with the wide bandgap of 6.2 eV. The piezoelectric coefficient d33 is about 5.5 pm/V and d31 is about −2.6 pm/V. The excellent electromechanical properties make it suitable for MEMS resonators. The film bulk acoustic wave resonators (FBAR) are widely studied and used. The resonant frequency of 1.8 GHz is achieved in FBAR. Another type of resonator, the contour mode resonator (CMR), is becoming an important area of research, achieving more and more attention. The resonant frequency of CMR can be achieved as high as 10 GHz. In addition, the Lamb wave resonators play an important role in MEMS resonators. This has some special advantages, such as the low motional resistance, high frequency and the multiple frequency capability. More research has been done by Chih-Ming Lin et al. With the development of wireless communication technology, the resonators should have higher resonant frequency and a higher quality factor value.
Piezoelectric AlN MEMS resonators have a “sandwich” structure: the bottom electrode layer, AlN layer and the top electrode layer. The substrate of these types of resonators is typically Si. In the manufacturing processes, the AlN etching is a key process. The etching result will strongly affect the performance parameters, such as resonant frequency and the quality factor. Recently, the inductively coupled plasma (ICP) etching process is widely used in the AlN etching process. However, there are still some problems in the process, such as poor sidewall angle, microtrenching effect and bottom roughness. Vladimir Bliznetsov et al. have done a lot of research in AlN etching. Their study shows the sidewall angle of 71.7°, the less obvious effects of microtrenching and the small bottom surface roughness of about 5–10 nm .
The etching sample in this experiment is polycrystalline AlN film with the thickness of 450 nm and the crystal orientation of (002). The full width of half maximum (FWHM) measured by X-ray diffraction is 1.7° and the roughness root-mean-square (RMS) is 3.2 nm measured by atomic force microscope (AFM). The grain size value is approximately 50 nm measured by scanning electron microscope (SEM). The hard mask of SiO2 with the thickness of 1 μm was used. In this paper, the selectivity is the etching rate ratio of AlN to SiO2. Chlorine-based gases were usually used in the etching process of AlN. The etching products are a series of Al-Cl volatile compounds, such as AlCl3, Al2Cl6 and other Al-Cl compounds. In this experiment, the Sentech etcher was used and the etching gases are Cl2/BCl3/Ar. In the etching gases, BCl3 was added to reduce the surface roughness. The initial process parameters are Cl2/BCl3/Ar = 25/15/5 sccm, ICP source power of 700 W, bias power of 80 W and chamber pressure of 0.5 Pa. The single variability method was used in the experiment. That means that only one parameter is changed and the other parameters are fixed in each recipe. The results were measured by SEM and AFM.
The etching rate and selectivity as a function of Cl2 flow rate are shown in Figure 1. The etching rate increases with the Cl2 flow rate. The value of etching rate is 47 nm/min when Cl2 flow rate is 20 sccm, whereas it increases to 110 nm/min with Cl2 flow rate 40 sccm. When other process parameters are fixed, the concentration of Cl atom and ion will increase with the increase of Cl2 flow rate. As a result, the chemical reactive etch will be enhanced, which will increase the etching rate.
The selectivity increases with the Cl2 flow rate too. It has the same trend with the etching rate versus to Cl2 flow rate. The selectivity increases from 0.5 to 1 when Cl2 flow rate increases from 20 to 40 sccm. In the etching process, the etching mechanism can be divided into two species, namely the chemical reactive etching and the physical bombardment etching. When the Cl2 flow rate is increased, the main mechanism of the etching process starts to deviate from the physical bombardment etching to chemical reactive etching. As a result, the etching rate of AlN film becomes faster. Whereas the etching rate of SiO2 is mainly decided by the physical bombardment etching, the etching rate of SiO2 mask is approximately independent of Cl2 flow rate. Thus, the selectivity increases with the increase of Cl2 flow rate.
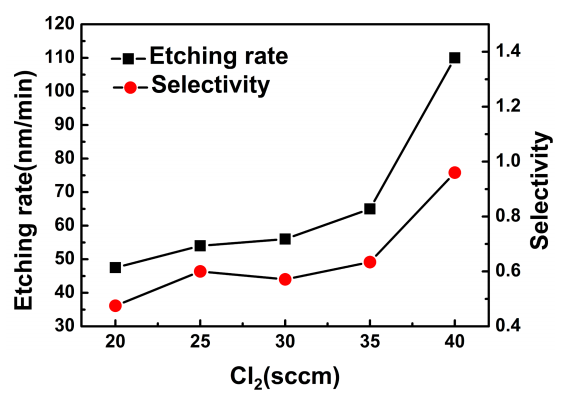
Figure 2 presents the etching rate and selectivity as a function of the chamber pressure. We note that the etching rates are shifted positively when the pressure is lower than 0.9 Pa, whereas negatively when it higher than 0.9 Pa. The chamber pressure is determined by the rate of inlet gas flow and outlet gas flow. A higher chamber pressure leads to a higher gas concentration. Thus, the etching rate increases with the increasing concentration of Cl atom and ion. However, the etching rate will shift downward with the pressure increased further. There are three reasons for this phenomenon. More collisions take place between the particles when the concentration is higher, which shortens the mean free path. The kinetic energy will decrease when the etching particles move to the surface of AlN film. This reason leads to a decreased etching rate. More collisions lead to more recombination between the etchant ions and atoms. This recombination will generate more stable molecules. Hence, the concentration of the active etchant will decrease. Due to the fixed rate of inlet gas flow, the outlet gas flow rate will decrease to meet the increase of chamber pressure. However, the etching products cannot be exhausted immediately. Some products will attach to the surface of AlN. The etching process will be suppressed to a certain extent. This also leads to the etching rate decreased.
上一篇: 可选择性去除氧化铝的湿式蚀刻法