Tribochemical polishing TCP has been applied to finish polycrystalline silicon carbide samples. With this technique, material is removed without intentionally using abrasives, by chemical dissolution stimulated by friction in a suitable reactive fluid. For the latter, Ž . oxidant solutions such as CrO , H O and KMnO were used. A smooth Ras1 nm , defect-free SiC surface is achieved when a SiC 3 22 4 sample is polished in a 3 wt.% CrO solution at speeds of 4–6 cmrs and loads of 1.96 N–9.8 N. The polishing rate is 3–7=10y6 3 mm3 rN m when rubbing against a Si N tool. Plane samples with surface dimensions of 2 cm=2 cm were polished by rubbing against 3 4 cast iron. The surface roughness was less than 1 nm, analyzed by atomic force microscopy. The polishing rate is 0.2–0.4 mmrh, which is comparable to that of the finishing step in ceramic lapping. The polishing mechanism of SiC is discussed in terms of interaction between mechanics and chemistry. q 1999 Elsevier Science S.A. All rights reserved.
When silicon nitride and silicon carbide slide in water, wear is tribochemical; the material is removed by a fricw x tion-stimulated dissolution in water 1 . This phenomenon has recently been used to develop a polishing method, w x called tribochemical polishing 2 . The technique consists in rubbing the piece to be polished against a suitable hard surface in the presence of a suitable liquid. No abrasives are used. This simple method can overcome many problems of surface damage and result in an ultra-smooth, damage-free surface of silicon nitride. The surfaces have a roughness less than 0.5 nm, are as clean as a cleavage w x surface and have residual stresses below 50 MPa .
Silicon carbide is much harder, more brittle and chemically more inert than silicon nitride, therefore it is more difficult to polish. In this study, the chemical and tribological conditions for tribochemical polishing of polycrystalline SiC are investigated in different oxidant solutions and the properties of the surfaces obtained are determined.
The SiC surface worn in dry sliding at the same speed Ž . Ž. 4.18 cmrs and load 3.92 N after 50 m sliding is shown in Fig. 3e for comparison. Besides the scratches existing on the worn surface, some grains wear much faster. The volumetric removal of SiC is not linear in dry sliding due to the accumulation of wear debris at the interface, which w x is well documented by other researchers 7,8 . The wear Ž . rate of silicon carbide at the beginning after 50 m sliding is 1=10y5 mm3 rN m, which is 2.5 times higher than that in CrO solution. The friction coefficient is 0.41.
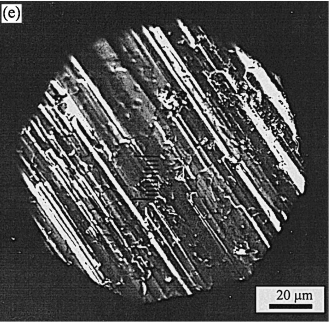
Fig3e
The polished surfaces are smooth in the whole range of variables: 2 cmrs to 6 cmrs speed and 1.98 N to 9.8 N load. No difference can be found by profilometer traces along the polished surface with magnification of 50,000. But the surface quality, which was carefully checked with Nomarski interference optical microscope and SEM, showed that there is an interplay between speed, load and the surface quality. At the low speed and high load, as in the case of load 9.8 N and speed 1 cmrs, some pitting occurs on the surface, even sometimes uneven grain polishing appears, similar to that obtained in hydrogen perox- Ž . ide Fig. 3b , on the polished surface. At the high speed and low load, surface quality is always good.
SiC samples with dimensions of 5 mm=5 mm were Ž polished against a Si N tool under a load of 37.6 N 1.5 3 4 . MPa at a speed of 4 cmrs. The original roughness of the SiC sample is 0.2 mm measured by profilometer. Fig. 5 shows a typical surface polished in the 3 wt.% CrO3 solution. At the beginning, the rough surface is smoothened at the asperities, as in Fig. 5a after 10 m polishing. With further processing, the surface is generally smoothened,but uneven polishing of the grains and mechanical scratches still remain on the surface, as in Fig. 5b after 50 m polishing. Finally, a well-polished surface is obtained, as in Fig. 5c after 500 m sliding distance. Profilometer traces on the tribochemically polished silicon carbide sample using 3 wt.% CrO and 20 vol.% H O solutions are 3 22 shown in Fig. 6. Tribochemical polishing by CrO yields 3 smooth surfaces and planarization. The surface roughness measured at different cut-off lengths is: in 3 wt.% CrO , 3 Ra F3 nm at 0.25 mm cut-off and Ra F4 nm at 0.8 mm cut-off; in 20 vol.% H O , Ra F5 nm at 0.25 mm cut-off 2 2 and Ra F7 nm at 0.8 mm cut-off. KMnO and distilled 4 water are not suitable for polishing large surfaces since scratches persist and the surface roughness is larger than 25 nm at 0.25 cut-off length.
Using cast iron instead of silicon nitride as a tool to polish large sample 2 cm Ž . =2 cm , one obtains even better surface quality. At a speed of 8 cmrs and load of 78.4 N Ž. Ž 0.20 MPa , the polishing rate is 0.2–0.4 mmrh corre- y6 3 . sponding to 3–6=10 mm rN m in the 3 wt.% CrO3 solution. A SEM image of a well-polished surface is shown in Fig. 7a. The pits on the surface are due to intrinsic porosity, as shown in Fig. 7b; no fracture or deformation was observed around these holes. The smaller black grains, shown in Fig. 7a, are sintering additives B C, 4 as determined by EDS. Further high magnification analysis revealed that the sintering additives are not well polished; some grains even have cleavage fractures, shown in Fig. 7c. The adjacent large silicon carbide grains are perfectly smooth. There is no evidence of plastic deformation and fracture on these grains. AFM images of polished silicon than 1 nm, as shown in Fig. 8b. The surface polished by H O is rougher and clearly shows the uneven polishing 2 2 of different grains, shown in Fig. 9a. With this feature, it still yields a smooth surface with a line roughness devoid of pores less than 3 nm, as shown in Fig. 9b.
The surfaces shown in Fig. 3a–c and Fig. 7 were obtained by rubbing the silicon carbide against silicon nitride in various chemicals. No wear particles were ob- Ž . served, in contrast to the case of dry sliding Fig. 3e . From this we conclude that the material removal was tribochemical, namely by a chemical dissolution that is stimulated by friction. Although several mechanisms can be imagined for the stimulation of chemical reactions by w x friction 9–11 , we do not have any evidence for a specific mechanism operating in the present case.