The shape control of nanoparticles constitutes one of the main challenges in today’s nanotechnology. The synthetic procedures are based on trial-and-error methods and are diffiffifficult to rationalize as many ingredients are typically used. For instance, concave nanoparticles exhibiting high-index facets can be obtained from Pt with difffferent HCl treatments. These structures present exceptional capacities when are employed as catalysts in electrochemical processes, as they maximize the activity per mass unit of the expensive material. Here we show how atomistic simulations based on density functional theory that take into account the environment can predict the morphology for the nanostructures and how it is even possible to address the appearance of concave structures. To describe the control by etching, we have reformulated the Wulffff construction through the use of a geometric model that leads to concave polyhedra, which have a larger surface-to-volume ratio compared to that for nanocubes. Such an increase makes these sorts of nanoparticles excellent candidates to improve electrocatalytic performance.
The properties of noble metal nanoparticles depend on their morphology and surface crystalline orientation and, thus, the specific control of the architectures is one of the major challenges in nanotechnology. Shape control has been achieved and several synthetic methods allow the production of selected polyhedra limited by convex surfaces (cubes, octahedra, tetrahedra, and rods).Many of the synthetic processes are based on the generation of a seed by a fast reduction of a metal salt, which is then grown on a solution containing halides and/or surfactants with a milder reductive agent. Recent studies have shown the effiffifficiency of biomolecules in recognizing specific crystallographic facets and affffecting nanocrystal shapes.The mechanism of shape control has been proposed to be kinetic or thermodynamic and is a consequence of the solution ingredients and conditions.In recent years, there has been considerable interest in the synthesis of concave nanoparticles (see Fig. 1). These structures have unique potential in electrocatalysis because of their exposed high-index facets, that encompass a larger surface area compared to that in convex systems and result in more active sites.In wet chemistry, two strategies have been proposed to generate concave nanocrystals of active metals: (i) directionally controlled overgrowth20,21 and (ii) site-specific dissolution.Overgrowth relies on the fact that capping agents, bound to specific facets, modify the relative growth rates leading to concave polyhedra. This is the case of Pt octapods exhibiting the facets, or Ag nanostructures that are formed when amines or Cu2+ ions are added.Octapods were also indicated to appear by kinetically controlled overgrowth in the case of Rh with bromide as a capping agent.
Halides and hydrogen halides form dense phases on metal surfaces. For Au and Ag, halides are employed as structure directing coatings that show a large preference for the {100} planes, and thus for cubes. This has been recently confirmed computationally, showing a thermodynamic control of the nanoparticle shape. Experiments on more reactive metals, like Pt or Pd, have found that hydrogen halides at certain concentrations lead to the formation of concave nanoparticles. In these cases, the process of the formation of high-index facets requires available low index-planes like {100} and {111} surfaces that serve as templates.
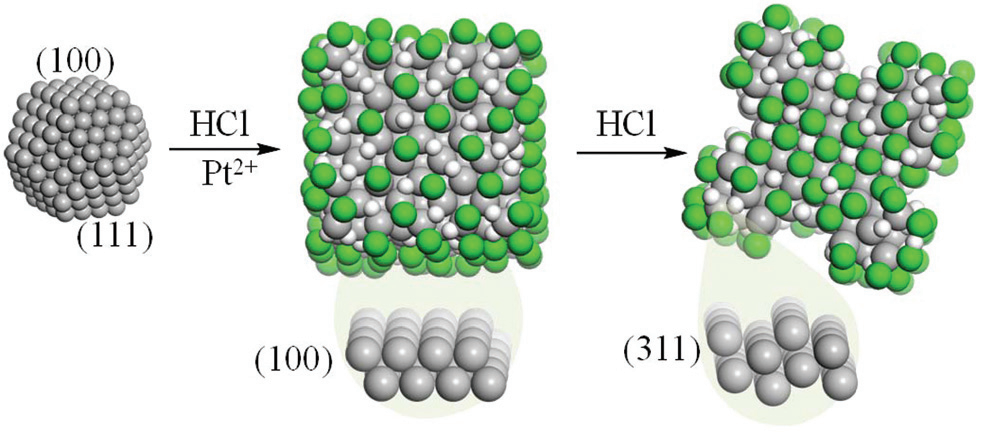
Fig1
To predict the shape of the nanoparticles in the HCl–water environment, we have adopted an ab initio thermodynamics framework,34 see section S4,† that allows the estimation of the surface energy for each of the surfaces as a function of the acid concentration, c. For this purpose, we considered the chemical potential of the species as the reference in solution at room temperature. The results for the surface energy for the HClcovered Pt surfaces, γHCl, at difffferent HCl concentrations are shown in Fig. 2 (left). The range of HCl concentrations corresponds to values between 0.5% and 100% (see Table S5†).
Theoretical calculations hold the key for an atomistic understanding of the control in the synthesis of nanostructures even when concave structures are generated. We have presented a formulation that describes the formation of concave Pt nanoparticles in the HCl–water environment. Our results based on ab initio thermodynamics predict cubic nanoparticles for any HCl concentration. However, at a certain HCl concentration, the gain for adding an extra Pt atom to the nanoparticle equilibrates with that of etching. Therefore, pitting starts at the most favorable elimination position, the facets. From this point, a geometric model based on the intersection of a nanocube and a polyhedron made of the {hll} planes predicts the equilibrium nanoparticle shape as a function of the degree of intersection, δ, between both the figures. When this parameter, which can be correlated with HCl concentration, adopts its maximum value, the structures are the octapods but resemble bonelike for smaller values as in the experiments. For the limiting case of octapods made of the {311} planes, the volume decreases 80% with respect to that for the cubic nanoparticle and the surface-to-volume ratio reaches a fold increase. The resulting insights advance the understanding of the role of additives during metal nanoparticle growth and etching and can be extended to other metals and structures, thus opening a new perspective in understanding the control of new shapes and architectures.