The infrared spectra of coesite, low-temperature tridymite, low-temperature cristobalite- low-~emperature quartz, vitreous silica, hexagonal Oe02, tetragonal Ge02, and vitreous germallla are reported from 4000 to 300 ㎝. Wherever possible an assignment of frequencies has been made on t he basls of the selectlOn rules for the crystal symmetry. Three characteris.tic gro up frequencies. near 1,lOq, 800, and 480 cm-1 are common to all the polymorphs of Sl02. Thes.e frequnCle.s respectlvely correspond to a strtching mode involving displacements assoclated prnnanly wlth the oxygen atoms, a stretclung mode involving displacements associated primarily . wi~h the. ilcoatoms, and a Si-O bending mode. The presence of these group frequencles III coeslte mdlcates that t he coordination of silicon in coesite is tetrahedral and that its high density is associated with the packinrr of tetrahedral units at an angle approximatiIg 120 degrees.. The tetragonal and hexagonl GC02 polymorphs show a marked dlfference III spectra due III part to the change from sixfold to fourfold coordination. The assignment of obscrved frequencies in hexagonal Ge02 is consistent with that made for low-temperature quartz if allowance is made for the heavier mass of t he Ge atom.
Several investigators have presented a detailed analysis of the vibrational spectrum of quartz. Infrared spectra of cristobalite and fused silica have been reported but no specific assignment of their spectra appear to have been given. Infrared spectra are presented of the Si02 polymorphs of coesite, cristobalite, tridymite, and vitreous silica; also the Ge02 polymorphs of hexagonal Ge02, tetragonal Ge02, and vitreous Ge02, in the region from 4,000 cm-I to 300 em-I. A partial interpretation of the spectra in terms of respective structures and selection rules is given. Particular emphasis has been placed on locating characteristic frequencies and on classifying the observed frequencies in terms of types of vibrations. The assiomnent of frequencies is admittedly tentative in part because either the true crystal symmetry is not known, the spectral data are incomplete, or the pectra are too complex. Because coordination of silicon in coe ite has not been established, one of the objectives of this work was to furnish evidence for either the fourfold coordination of the other polymorphs of Si02 or for a different coordination. In the tetragonal form of Ge02 the germanium atom has a sixfold coordination with oxygen, whereas in the quartz-type hexagonal Ge02 the germanium atom has the fourfold coordination of silicon in quartz . A similar tetragonal form of silica is not known but has been predicted by analogy with germania.
A Si02 group in a rigid framework has 3N-3 or SLvibrational degrees of freedom. There is some ambiguity as to how the corresponding modes of vibration should be classified in terms of bond stretching, bond bending, and bond-distortion types. Somewhat arbitrarily a bond-stretching and bondbending mode will be associated with each 0 atom. These O-stretching modes should correspond to the highest observed frequencies in the spectrum. Two remaining degrees of freedom need to be assigned to the Si atom. One of these must be a stretching mode corresponding approximately to motions of the Si atom between the two 0 atoms. The one remaining mode must correspond to a low-frequency distortion or bending motion of the Si atom.
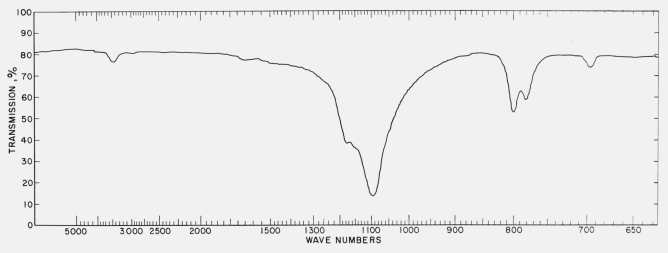
Fig1
The feasibility of the method outlined above for the classifications of the normal modes of various forms of silica may be tested on the assignment given for quartz by Saksena. From table lone would expect, in order of decreasing frequency, four distinct spectral groupings consisting of SL Si-O stretching modes involving displacements associated primarily with the 0 atoms, five stretching modes involving displacements associated primarily with the silicon atom, six Si-O-Si bending modes and seven low-frequency distortion modes. These are readily associated with the frequencies observed in the following regions of the spectrum: 1,200 to 1,000 cm- I, 825 to 600 cm-I, 600 to 390 em- I, and 380 to 100 cm-I, respectively. The distinction between the latter two groupings is somewhat arbitrary. If one allows for the fact that frequencies assigned to species E in table 2 must be counted twice because they are doubly degenerate, it is found that these groupings contain 6, 5, 6, and 7 degrees of freedom, respectively, in agreement with the numbers expected from a unit cell containing three Si02 groups.
The observed spectra were taken on low-temperature a -cristobalite. Since little information is available about its structure, the spectra are interpreted on the basis of that for high cristobalite for which more structural information is available . High cristobalite was studied by Wyckoff for which he proposed a cubic structure of space-group symmetry Or= Fd3m with eight Si02 groups per unit cell. Application of Oh selection rules shows that only 5 triply degenerate fundamental frequencies of species F III corresponding to 15 of the 69 modes of vibration for the unit cell should appear in the infrared spec- trum. Because the observed spectrum of low-temperature cristobalite contains at least seven frequencies plus additional low-frequency peaks below 300 cm- I , it is not feasible to interpret the infrared spectrum on the basis of Or= Fd3m space-group symmetry. Barth reexamined the structure of high temperature ,B-cristobalite and concluded that its structure corresponded to a lower symmetry and proposed a structure with space-group symmetry T4= P213. Application of T-selection rules to a unit cell with eight Si02 units predicts that seventeen triply degenerate fundamental frequencies should be observed in the infrared spectrum. At least twelve of these should be observed in the region above 300 cm-I , and it is consider ed unlikely that a satisfactory interpretation of the spectrum can be made on the basis of space-group symmetry T4= P213.