The bonding energy of bonded native-oxide-covered silicon wafers treated in the HNO3 /H2O/HF or the HNO3 /HF solution prior to room-temperature contact is significantly higher than bonded standard RCA1 cleaned wafer pairs after low-temperature annealing. The bonding energy reaches over 2000 mJ/m2 after annealing at 100 °C. The very slight etching and fluorine in the chemically grown oxide are believed to be the main contributors to the enhanced bonding energy. Transmission-electron-microscopic images have shown that the chemically formed native oxide at bonding interface is embedded with many flake-like cavities. The cavities can absorb the by-products of the interfacial reactions that result in covalent bond formation at low temperatures allowing the strong bond to be retained.
The bonding energy can reach about 1.2 J/cm2 after annealing at 150 °C.3 In order to obtain a stronger bond for applications such as leak-free sensors, usually annealing above 1000 °C is necessary. However, the high temperature treatment is not compatible with bonding of processed silicon wafers that are thermally sensitive. Therefore, it is highly desirable to develop methods to yield a strong bond at low temperatures.
Several different methods have been developed for low temperature bonding of bare silicon wafers. A surface treatment of plasma of oxygen or other gases in reactive ion etch mode prior to room temperature bonding can significantly increase the bonding energy at low temperatures.4–6 It has been reported that an oxygen plasma treatment of nativeoxide-covered silicon wafers can increase the bonding energy to about 1500 mJ/m2 at room temperature. However, the plasma treatment introduces a damage layer in the near surface region that may degrade the device performance.Although a dip in concentrated 70% nitric acid can enhance the bonding energy of bonded bare silicon wafers at low temperatures, many voids developed at the bonding interface upon annealing due to the release of the trapped gaseous nitrogen monoxide in the chemically grown oxide.
In this letter, we report the use of the mixtures of an oxidizing agent s HNO3d and an oxide-etching agent (HF) to treat bare silicon wafers prior to bonding. Bonding energy close to the bulk silicon fracture energy has been obtained after annealing at 100 °C.
It is known that at a given annealing temperature, the bonding energy of a bonded hydrophilic silicon wafer pair reaches its saturation value after a certain period of time. For the HNO3 /H2O/HF treated bonded pairs the bonding energy at 100 °C has reached over 1000 mJ/m2 after annealing for 5 h and is strong enough for many applications. The bonding energy reaches its saturation value of , 2.4 J/m2 in , 20 h, and the wafers were usually fractured when inserting a razor blade at the crack opening measurements.
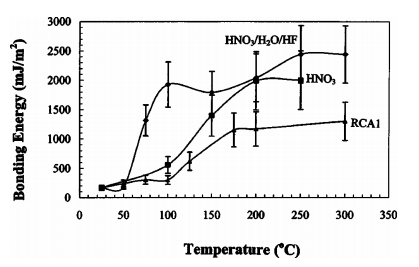
Fig1
In summary, the bonding energy of bonded bare silicon wafers treated in the HNO3 /H2O/HF or the HNO3 /HF solution prior to room-temperature contact reaches silicon fracture energy after annealing at 100 °C. The very slight etching and fluorine in the chemically grown oxide are believed to be the main contributors to the enhanced bonding energy. TEM images have shown that the chemically formed native oxide at bonding interface is embedded with many flake-like cavities. The cavities can absorb the by-products of the interfacial reactions that result in covalent bond formation at low temperatures allowing the strong bond to be retained.
下一篇: 硅太阳能电池的红外线研究