In recent years, the formation of microstructures on silicon wafer has gainedpopularity as a concept for increasing photon trapping and light absorption foroptoelectronics applications. This study used three methods to improve infrared lightabsorption in silicon samples - sample preparation, Radio Corporation of America(RCA) cleaning, and chemical wet etching. The solutions used for Radio Corporationof America (RCA) clean were water (H2O), Ammonium hydroxide (NH4OH),hydrogen perioxide (H2O2), Hydrofluoric acid (H.F.). Three silicon wafers with a1cm2 orientation were cut and cleaned using RCA, and then surface-textured using awet chemical procedure by etching into different chemical solutions of Sulfuric acid(H2SO4) of the same concentration. The wafers were removed at different etchingtime intervals (5, 10, 15 minutes) and analysed using an infrared spectrometer withFourier transformation (FTIR) to study the absorptions of light. A mean absorbanceof 0.9801 a.u, 0.9845 a.u and 0.977 a.u for 5, 10 and 15 minutes of texturization wasobtained. The results showed a wafer that was etched by H2SO4 solution for 10minute as the most enhanced silicon wafer for I.R light absorption. Hence, it isrecommended to texture a silicon wafer for a period of 10 minutes in H2SO4 solutionfor better absorption.
The ratio of energy output from the solar module toenergy input from the sun is known as a photovoltaicmodule’s efficiency. It measures the proportion ofsolar energy shining on a P.V module that istransformed into usable power. Enhancing lightabsorption is a key goal of this research. Efficiency isa factor used to compare the performance of varioussolar modules. The solar module’s efficiency isinfluenced by the solar panel’s temperature and thespectrum and intensity of the incident sunlight.Hence to improve the device’s performance underthe conditions used to measure efficiency, the solarradiation must be appropriately captured bytexturing the silicon wafers surface. None of thereviewed paper in this research find out the absorption of I.R light by the silicon wafers beforefabricating solar cells and the best etching time whenusing sulfuric acid as an etchant (even though nobodyhave ever work with sulfuric acid as an etchant inimproving light absorption by the silicon wafer) andnone of the authors cited in this research found outlight absorption but only worked on the lightreflections, by knowing the intensity of light beenabsorbed even before fabricating solar cells one cantells the silicon wafer that would come out with thebest solar cell performance efficiency because highabsorption of light causes less reflection of light bythe silicon wafer and this result in increasing theefficiency of any solar cell. Solar modules efficiencydepends on its active area and the intensity of light shining on it. That is the more direct irradiance themore efficiency the module will produce as theefficiency is given by the ratio of the maximumpower (short circuit current (Isc) multiply by opencircuit voltage (Voc)) to the ratio of the directirradiance multiply by active area. This clearly showsthe relationship between these four (4) factors asmentioned above to the efficiency of any solarmodule. This research work created a means ofattenuating the reflectance of light shining on thesurface of the silicon wafer so that more light is taken(absorbed) by the silicon wafer as this will increasethe efficiency of the solar module when fabricated.
In a study conducted by Liman et al., (2014) studiedthe attenuation in the mid-infrared transmissionsthrough poly (methyl methacrylate) PMMA film ofdifferent layer thickness on SiO2 substrates for solarcell applications using Fourier transform infraredspectrometer. The experiment was conducted on foursamples one without PMMA coat and the other threecoated with PMMA film layer at different rotationalspeeds. The film thickness decreases with the increasein rotational speed and the infrared transmissiondecreases with the increase in film thickness. Hightransmission are detected at the range of wavenumber between 2900 cm-1and 7800 cm-1. Thesample coated with 250nm thick layer of PMMA filmhas the highest infrared transmission at 7800cm-1while the sample coated with 480.8nm have thelowest infrared transmission at the same wavenumber. Numerically, for an uncoated sample, at7800 cm-1 the transmission efficiency of 92.14% wasobtained, while for a sample coated with 250nm layerof PMMA at the same wave number 92.84% wasrecorded. Meanwhile for sample coated with 274.4nm and 480.8 nm layers of PMMA, the transmissionsof 88.40% and 60.69% were respectively obtained at7800 cm-1(Liman, et al., 2014). In a study conductedby Yasir et al., (2021) With reference to photontrapping and increased light absorption qualities forsolar applications, the synthesis of black silicon viasurface texturization of Si-wafer has recently gainedpopularity.
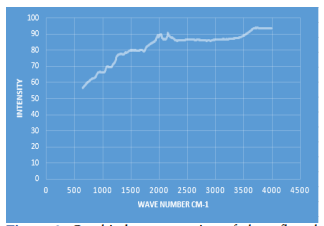
Fig1
Regarding photovoltaic (P.V.), silicon is utilized asthe raw material for mono-crystalline and multicrystalline wafers and for thin-film silicon modules.crystalline silicon wafers are the foundation of morethan 90% of the yearly manufacturing of solar cells.The most significant component of photovoltaic(P.V.) technology today is silicon (Muller et al., 2006);although multi-crystalline silicon wafers arefrequently utilized in the fabrication of commercialsolar cells, they typically produce cells withsignificantly less performance than mono-crystallinewafers (Zhao et al., 1998).
The conical flask was utilized in hosting the chemicalsolutions during the process RCA clean and texturingof the silicon wafers, a burette is a volumetricglassware which was used in this research for theaccurate dispensing of the chemical solutions insidethe conical flask, retort stand is a piece of scientificequipment in supporting other pieces of equipmentand glassware, the measuring cylinder is a commonpiece of laboratory equipment used to measure thevolume of the chemical solutions in this research, hotplate was used in heating of the chemical solutions tothe required amount of temperature while thethermometer was used in measuring the temperaturesof the chemical solutions.