It is well known that in semiconductor materials and device fabrication, the important issues are materials’ purity, growth, defectcontrol, lithography, etching, contact formation, and device performance.1,2 Among these, etching is an important process in whichthe grown material is required to be partially removed using specificpatterns for subsequent doping, metalization, device structure formation, etc. In such processes, the rate of material removal has to befast and repeatable. There is another associated process, in whichthe top layer of a material is cleaned before subsequent growth byremoving a few angstroms and associated top layer contaminants. Inthis cleaning process, it is essential that the top layers are removedwith care and the least amount of material is wasted. Thus, thischemical cleaning process has to be one with a slow material removal rate and with the least amount of interfering residues.
Such etching and cleaning may be done by electrochemical, wetchemical methods, reactive ion or sputter ion cleaning.3 Electrochemical processes are not always very selective while removingmaterials and often require very tight control over bias voltages andcurrents. Wet chemical etching is a method of removing the toplayer of a material in a solvent leading to its dissolution in the reaction medium. Often the etchants are a combination of acids or acombination of bases. In wet chemical methods, with proper calibration, it is possible to retain better material crystalline quality of theetched material and by controlling the concentration of chemicals inthe reaction, the material removal rate can also be rigorously controlled. However in all etch steps, residues from etching steps arepresent; these need to be understood to evaluate the likely effects onthe subsequent electrical properties of the device created. Wetchemical etching is thus often the preferred etching mechanism forlithographic process. It has often been seen that the traditional wetchemical or electrochemical processes may also have their otherlimitations, even though they are quite cost effective. Inability to gethigh etching rates or reaction induced surface passivation are someof the associated problems. Reactive ion etching and sputter ioncleaning, also called dry etching, was developed to overcome suchproblems. These processes have relatively higher etch/erosion rates,depending on the material being removed and the reactive or sputtering gas used. But they also have their own limitations in terms ofhigher defect and crystalline damage creation and residue implantation. Over the years, this dry etching procedure, has often becomethe standard operating procedure for certain specialized semiconductor industries.4–6 Interestingly, wet chemistry continues to playan important role with RCA cleaning, chemical-mechanical polishing and copper deposition.
GaN and and associated materials are quite hard and reports oftheir wet etching are few,7 with most reports preferring the dry method, even though it requires large capital investments.4 However, the reported sputtering rates for GaN and related materials arealso quite low and thus sputter etching is also time consuming.Photo-chemical and light induced electro-chemical etching techniques are other etching alternatives currently available for InGaN/GaN related materials.8 In this context, we discuss the possibilitiesof etching InGaN/GaN type materials using wet chemical methods.The choice of acidic and basic mixtures to be used is an issue whenan unknown material is to be wet chemically etched. There wereliterature reports on the use of etchants like (i) mixture of hotconc. H2SO4 and H3PO4 at 250C (Ref. 9); (ii) HCl; (iii) NH4OH(Ref. 10); but the usage and performance analysis of these last twoin succession to combine the oxidizing and then the reducingfeatures together was not envisaged earlier; that has been done here;(iv) usage of HF and HNO3 mixture based (isotropic and anisotropic) etchants was based on one of the authors’ past experience ofworking on GaSb based materials.11 The etching performance ofthese chemical mixtures were then compared. X-ray photo electronspectroscopy (XPS) of the etched samples were performed to knowthe chemical contaminants present after the etch so that futureresults of electrical measurements can be appropriately correlated.This also gave information on the chemical bonding environmentsfor Ga, In, N, and O on the surface. Such an analysis, using the similarities of an earlier report12 will also help assign the XPS spectra.
GaN and InGaN samples were prepared by organo-metallicchemical vapor deposition/epitaxy (MOCVD/MOVPE) process,with sapphire (Al2O3) as a substrate material. The growth details arebriefly the following: a base layer of undoped GaN was grown onthe sapphire to a thickness so that the surface growth defect densitystabilized and epitaxial layer growth was achieved. A semi-insulating GaN layer was grown on top of this layer to isolate future electronic structures. The total GaN layer thickness was at least 2000nm or more. Often the GaN thickness was double of this. This bufferlayer was required to get an appropriate lattice matching for thesubsequent InGaN/GaN based active layers. Active semiconductorlayers based on p and n type InGaN were grown on top of this(Fig. 1).13 The In content was about 17% or so. This active layerthickness varied from 150 to 450 nm or so, depending on the type ofdoping profile and structure needed.
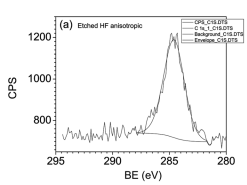
Fig1
The uniqueness of this etch was that four Ga 3d5/2 peak components were visible at 18.4, 19.1, 19.8, and 20.9 eV possibly corresponding to Ga-N, Ga-O, Ga-(NO3)3/GaONx and Ga-F/oxyfluoride(Ga-OFx) respectively (Fig. 4b). There are also two oxygen peaks tosuggest possible presence of NO3. The Ga-(NO3)3/GaONx peakswere visible separately due to the higher NO3 ion content in the etchsolution. A comparison of the relative peak intensity changes for thedifferent peak components related to Ga 2p3/2 and Ga 3d5/2 showsthat the top layer contains relatively more intense peaks for theoxides while at the more deeper parts of the sample, the nitride peakcomponent is predominant.