Etching of monocrystalline silicon in alkaline based solutions leads to adeep minimum in the etch rate crystallographically oriented along h111i. Thedetails of the form of the minimum (angular dependence of the etch rate) areinvestigated and discussed in a framework of steps originating from spontaneousnucleation and from misorientation of the crystal face exposed to the etchant. As aresult, the etch rate minimum is characterized by a narrow flat portion that reflectsthe density of nuclei, and the temperature dependence of the width has anactivation energy equal to 1/3 of the nucleation barrier.
This article deals with the dependence of the siliconetch rate close to the h111i orientation when etchingin anisotropic etching solutions such as aqueous KOHand EDP‡. In these solutions the etch rate is stronglydependent on the crystallographic orientation of the siliconsingle crystal, with absolute minima in h111i, and relativeminima in h001i (aqueous KOH) and h110i (aqueousKOH saturated with isopropyl alcohol, IPA and EDP).An extensive discussion on experimental results can befound e.g. in [1]. The origin of the anisotropy is beingdebated in the literature. While many authors try to findexplanations based on (electro-) chemical reactions of wateror hydroxyl ions with the silicon surface for orientationdependent contributions [1–4], this author proposed to usemodels developed for the rate of crystal growth to find theorigin of the anisotropy [5–7]. This view has consequenceson some details of the etch rate diagram (i.e. the variationof the etch rate with the crystallographic orientation) thatwill be explored here and compared to experimental datataken from the literature. In our view, understanding theanisotropy of the etch rate of silicon (and other singlecrystalline materials, such as quartz and GaAs) is notonly interesting from a scientific point of view, but alsofor the development of micromachining. Wet chemicaletching is a key technology for micromachining because itallows fast, precise and reproducible shaping of mechanicalmicrocomponents such as numerous sensors for pressure,force, acceleration, flow, temperature and concentrationof chemicals, actuators like pumps, valves, switches andmicrostructures such as microsieves, canals and nozzles.The major volume is currently being fabricated in research laboratories, but scaling up to mass production makesperfect control of the technology necessary, which isvery difficult to achieve if the origin of a prominent andimportant property of the etching—the anisotropy—is notunderstood. This is a quite formidable task, and this paperwill contribute a step towards executing to it.
Starting with a short outline of the crystal growthview on wet chemical anisotropic etching, we focus onthe kinetics of steps on misoriented flat crystal faces. Wehave found that there are two competing mechanisms forthe production of steps on crystal faces of perfect crystals(spontaneous formation of steps by two-dimensionalnucleation§ and the steps due to misorientation). Thepeculiar interaction of steps leads to a picture in which themore effective step source dominates the etch rate, resultingin a very interesting shape of the minimum. This shapehas been found by Uenishi et al [8] for Sih111i. Earlierresults [1, 9] agree with Uenishi’s results but due to a muchsmaller angular resolution, this particular shape was notapparent. Similar results, but less pronounced, are reportedfor Sih001i [6].
Flat faces can often be found from the crystallography,however, in the diamond lattice, crystallography providesus with only the h111i face as a flat face. Other faces canbe flat due to additional surface effects such as adsorptionand surface reconstruction. We ignore these effects here,because we concentrate on the h111i which does not seemto be reconstructed, at least when etching in NaOH at roomtemperature.
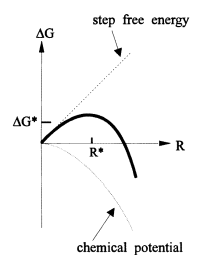
Fig1
This dependence changes if the distance betweenthe steps becomes wide, i.e. if 2 becomes very small.Eventually the density of nuclei between the steps becomesso large that the total length of steps due to themisorientation becomes smaller than the length of the stepsdue to nucleation.
上一篇: 化学蚀刻的硅:IV蚀刻技术
下一篇: 硅纳米加工中KOH湿法蚀刻工艺的优化