Abstract: A nano-multilayer Ti0.2Al0.55Cr0.2Si0.03Y0.02N/Ti0.25Al0.65Cr0.1N PVD coating was deposited on Kennametal carbide K 313 inserts. These coatings are widely used to protect cutting tools under severe exploitation conditions. Under equilibrium conditions, it was found that the Al2O3 oxide possessed better adhesive properties than the TiO2 . The addition of chromium further enhanced the oxidation resistance of the coatings. Silicon significantly increased the oxidation resistance of this type of coating. The properties of the diffusion process in this coating have not been sufficiently investigated, despite the considerable number of articles published on this topic. For the purpose of this study, a multilayer ion-plasma (TiAlCrSiY)N/(TiAlCr)N coating was oxidized under equilibrium conditions; its chemical inhomogeneity was studied by time-of-flight mass spectroscopy using a TOF SIMS5-100 instrument. The data was collected from an area of 100 × 100 µ. A D-300 profilometer (KLA-Tencor Corp., Milpitas, California 95035, USA) was used to determine the rate of ion etching. It was found that oxidation commenced at the surface nanolayer of a TiAlCrN nitride, forming loose films of Cr2O3 , TiO2 , and Al2O3 oxides. This passivating film had a thickness of around 140 nm. For the first time, the interlayer diffusion coefficients of Si and Y were determined in multilayer coatings based on Ti0.2Al0.55Cr0.2Si0.03Y0.02N/Ti0.25Al0.65Cr0.1N, under open air annealing at 700 ◦C. The physical nature of the differences in the diffusion of these elements is discussed. The diffusion rate in the near-surface volumes was lower than in the deep layers of the multilayer coating, most likely due to the formation of passivating oxide films on the surface.
Introduction
Mono- and multilayer TiAlCrN-based coatings with inclusions of Si and Y have been intensively studied for machining applications. These wear-resistant coatings have high hardness, wear resistance, and thermal barrier properties .
The oxidation of such coatings deserves attention for several reasons: The first is due to the need for protective, heat-resistant materials—including cutting tools that operate under aggressive external conditions. In this case, the coatings form oxide films, which serve as diffusion barriers against aggressive chemical elements that cause metal degradation.
Materials and Methods
A multilayer Ti0.2Al0.55Cr0.2Si0.03Y0.02N/Ti0.25Al0.65Cr0.1N coating was deposited using Ti0.2Al0.55Cr0.2Si0.03Y0.02 and Ti0.25Al0.65Cr0.1 1 targets, correspondingly fabricated by a powder metallurgical process on a cemented carbide cutting insert WC-Co substrate in a R&D-type hybrid PVD coater (Kobe Steel Ltd., Onoecho Ikeda, Kakogawa, Hyogo, Japan) using a plasma-enhanced arc source. The WC-Co samples were heated to 500 ◦C and cleaned by Ar-ion etching. During the PVD process, an Ar–N2 mixture gas was fed into the chamber at a pressure of 2.7 Pa with a nitrogen partial pressure of 1.3 Pa. The arc source was operated at 100 A on a 100 mm diameter × 16 mm-thick target. The other deposition parameters were a bias voltage of 100 V and a substrate rotation of 5 rpm. The thickness of the studied coating was around 3.5 µm for the film characterization. The coating had a columnar nanocrystalline multilayered microstructure with alternating nanolayers at every 50 nm, a hardness of 30 GPa—according to the nanoindentation at room temperature—and 28 GPa at 500 ◦C.
Results
The coating had a complex structure that combined a nano-multilayered structure with a modulating composition with a columnar structure (with a grain size of around 5–20 nm).
Figure 1a presents a HR TEM image of the TiAlCrSiYN layer. The coating had an ultra-fine nano-grain size in as-deposited conditions. The grain size of the coating was within 5–10 nm.
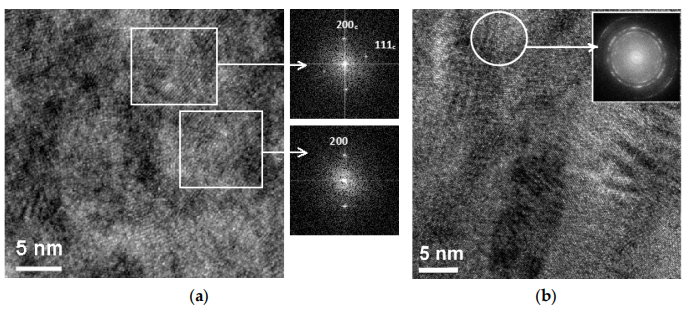
Figure 1. TEM image of the (TiAlCrSiy)Nlayer (a) and the (TiAlCr) layer (b) of the nanolaminatecoating
Figure 1b presents a zero-loss HRTEM image of the coating. The image does not showepitaxial growth between the layers. The layers were polycrystalline and did not have apreferred orientation, as can be seen in the ring diffraction pattern.
After annealing the samples in an open furnace, a layer of oxides appeared on thesurface of the coating, which had a porous, layered microstructure. Mass spectroscopymade it possible to establish the phase composition of the oxidized surface. Figure 2 shows that complex oxide films were formed on the sample surface during heat treatment;these were oxides of chromium and titanium. Similar phases appear under conditionsof high-speed dry cutting . Mullite-like (AlSi-O) tribo-films with a high protectiveability are also formed . The depth distribution of chemical elements and phases wasevaluated through cyclic etching of the surface with Cs* and Bi' ions. During active surfaceetching with cesium ions, layers of atomic thickness were sequentially removed. Etchingwith bismuth ions in the static mode during pauses excited a flow of scattered secondaryions, which were analyzed in a mass spectrometer. Such complex surface etching made itpossible to represent the deep distribution of the chemical elements.
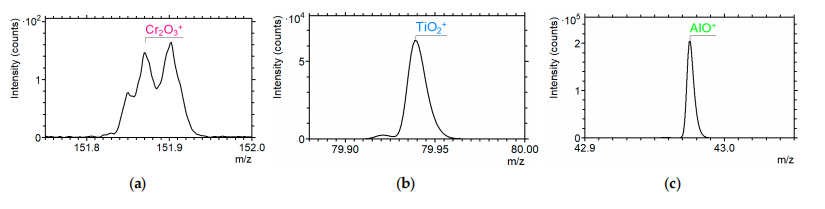
Figure 2. Mass spectra of secondary Cr20;* (a), TiO,* (b), and AlO+ (c) ions from the TiAlCrSiYN/TiAlCrN coating after annealing in an open furnace for 45 min at 700 °C, obtained at differentdepths from the surface during ion etching.
These data provide a basis for a model of the distribution of phases across the depth ofthe oxide film, presented in Figure 3. Si and Y were not found within the equilibrium oxidefilms. After a 45 min annealing process, the oxide film had a thickness of about 140 nm. The1.5 of the first bilayer was oxidized. Computer processing of the data obtained in each layerduring surface ion etching made it possible to reconstruct a 3D picture of the distributionof the volume phases.
Conclusions
1、The coefficients and rates of diffusion of the chemical elements of multilayer thinfilms were calculated on the basis of their depth distribution profiles. Secondaryion mass spectroscopy was used to obtain depth profiles with a high sensitivity anddep th resolution.
2、The effective diffusion coefficients of silicon and yttrium were determined in a multilayer nanolaminate Tig2Al.55 Cro2Si03 Yo02N/Tip.25Al.65 CroN coating after anneal-ing at a temperature of 700 ·C in air.
3、It had been established that the diffusion rate of Si was several times-higher than thatof Y, which can be associated with a significant (about two times) difference in thesizes of the atoms between these elements.
4、It had bee17found that the diffusion rate in near-surface volumes was lower than in thedeep layers of a multilayer coating most likely due to the formation of passivatingoxide films on the surface.
5、An anomaly of the preferential diffusion of Si in at-surface volumes has been established; this may be due to the lower stability of metal-nitride atomic bonds andthe greater affinity of Si for oxygen compared to Y. These assumptions require theirexperimental or theoretical erification in the future.
搜索“华林科纳行业观察”小程序查看完整内容,或者加微信13358064333/18106288187。