Cleaning is one of the key steps of the integration of self aligned barriers (SAB) in microelectronic devices for 32 nm technology and below. It is hence important to investigate the impact of different cleaning solutions on the metallic components of SAB, mainly in which concerns their surface stability. In this sense, the electrochemical behavior of copper and cobalt was studied under potentiodynamic conditions in different aqueous solutions of glycolic acid (GA) with and without benzotriazole (BTA) inhibitor. It has been observed that the presence of glycolic acid induces a monotonic increase of copper corrosion and a slight decrease in the case of cobalt. The cobalt dissolution remains nonetheless very active and is shown to be governed by oxygen reduction reaction. The addition of BTA, a well-known corrosion inhibitor for copper has shown to be also effective in the case of Co surfaces, with a ca 15-fold reduction of the intrinsic Co corrosion current density. The possibility of galvanic coupling between both metals, supposed to enhance the Co dissolution, has also been qualitatively investigated and seems not to be a determinant factor in these conditions.
The copper damascene process is widely established and has brought higher performance to semiconductor devices [1]. However, due to continuously shrinking design rules and hence to increasing current densities, copper electromigration remains an important challenge. To overcome this issue, the integration of CoWP or CoWB self aligned barriers (SAB) for 32 nm technology was proposed [2–5].
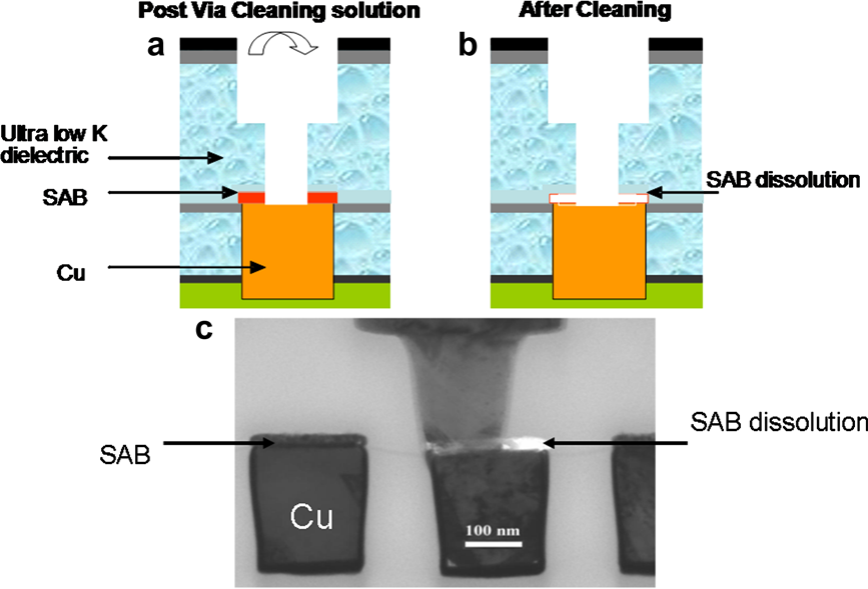
The integration of these new materials implies a compatibility study with the various conventional dry and wet treatments used for the patterning of interconnection levels. Particularly, the postvia etch cleaning can strongly impact the integrity of the SAB due to corrosion. In the ideal integration scheme, the SAB is etched in order to minimize the resistance of vias [6]. In this confifiguration, the copper at via bottom is in contact with the SAB and both are exposed to the cleaning solution (Fig. 1a).
This may lead to SAB dissolution not only by spontaneous corrosion processes, but also those induced by galvanic coupling as schematically illustrated in Fig. 1b. Indeed, the simultaneous presence of dissimilar metallic components exposed to an ionic conductive medium can entail galvanic corrosion processes yielding accelerated damage of the less noble of the metallic phases during the post-via etch clean step [7]. The standard potentials of the Cu/Cu2+ and Co/Co2+ couples are ECu=Cu2þ ¼ +0.34 V/ENH, and ECo=Co2þ ¼ 0.28 V/ENH, respectively. Thus cobalt is expected of undergoing galvanic corrosion when is in contact with copper, enhancing its selective dissolution as it clearly appears in Fig. 1c. On this TEM picture a total withdrawal of Co-alloy after the via opening and the cleaning step is evidenced.
上一篇: 超细金属颗粒在硅片表面的行为
下一篇: 晶片光刻胶处理系统