Removal of particulate contaminants from surfaces is a critical area in the processing of wafers to build integrated circuits and is typically done using wet chemical formulations. Strategies for particle removal require an understanding of particle deposition mechanisms. In the fifirst part of this review paper, colloid chemical principles underlying particle deposition on surfaces is discussed. Specififically, the importance of van der Waals and electrical double layer interaction energy on particle–surface interaction is reviewed. This is followed by a description of commonly used particle removal methods in wafer processing, viz., megasonic cleaning, brush cleaning, and nanospray cleaning. The importance of particle deposition conditions used in research on particle removal effificiency is highlighted through a study on the effect of aging.
Keywords: megasonic cleaning, particle deposition, particle removal, wet processing of wafers
Introduction
Removal of small particles from surfaces is an area that has great technological implications. In integrated circuit (IC) manufacturing, as the fabricated size of features on wafers is decreasing to below 20 nm, particles which are smaller than this dimension can be defects that adversely impact the yield and reliability of fabricated devices. Because of the large number of wet chemical processing steps used in manufacturing, particle contamination from liquid chemicals and deionized (DI) water is of major concern. In some instances, particles may also be generated in situ. Interestingly, a widely used process known as chemical mechanical planarization (CMP) uses a slurry of particles to planarize wafers. While this process defifies the very concept of avoiding particles during manufacturing, thanks to post-CMP particle removal strategies, this process has become a viable technology.
Advances in methods for reduction of extent of deposition as well as effective removal of particulate contaminants have been made through the utilization of principles of surface and colloid chemistry. The classical particle removal method practiced in wafer processing uses an aqueous formulation containing ammonium hydroxide, hydrogen peroxide and water. This formulation, knows as SC-1 or ammonium hydroxide hydrogen peroxide mixtures(APM), relies on disrupting the adhesion of particles to surfaces by controlled etching of the surface and by preventing the re-deposition of particles by a negative surface charge both on the particles and surface that leads to electrostatic repulsion. With increasingly stringent restrictions placed on the extent of allowed etching of surfaces during processing, the semiconductor industry has been challenged to develop more innovative approaches. The objective of this paper is to review principles and methods of particle deposition and removal of interest to semiconductor processing. After a discussion on van der Waals (vdW) and electrical double layer forces of interaction, the paper discusses methods for particle removal.
Energy of Interaction between Surfaces and Particles
Van der Waals Energy For the case of the interaction of a particle with a flflat surface in a liquid medium, the vdW energy of interaction is given by
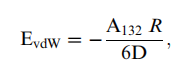
where R is the radius of the particle, A132 is the effective Hamaker constant between particle 1 and surface 2 immersed in a medium 3, and D is the closest distance of separation between the particle and the surface. The effective Hamaker constant can be calculated from the individual Hamaker constants, A11, A22, and A33 using the following relationship:
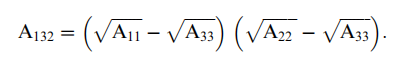
Values for Hamaker constants for different materials of interest to semiconductor manufacturing, in air (or gas) and in water are given in Table 1. The most common method to theoretically estimate Hamaker constant is based on Lifshitz theory (Liftshitz, 1956; Dzyaloshinskii, Lifshitz, and Pitaevskii 1961). Hamaker constant can also be calculated from atomic force microscopy (AFM) measurements using the colloidal probe technique (Kappl and Butt 2002). In this technique, the particle of interest is mounted to a tipless AFM cantilever, which is then brought into contact with the substrate of interest while measuring the force at the same time. Figure 1 shows a typical force versus distance curve during AFM measurement (Kappl and Butt 2002). During approach of the probe to the surface of interest, the colloidal probe is far from the surface (stage 1), no deflflection of the cantilever occurs and thus no interaction force is detected,and this segment is regarded as zero-line. When the probe gets closer to the surface, cantilever may bend downward toward the surface if the interaction force is attractive (stage 2). When this attractive force exceeds the spring constant of the cantilever, as the probe continues to approach the surface, the probe will jump into contact with the surface and move together with the surface (stage 3). During retraction, the probe stays in contact with the surface due to adhesion force (stage 4) until it jumps back to its original undeflflected position (stage 5). Hamaker constant of a system can be calculated from the ‘‘jump-in force’’ (Stage 2 in Figure 1) or from the ‘‘pull-off’’ (adhesion) force, which is designated as FA in Figure 1. The effect of geometry and roughness of particle and surface on interaction force has been modeled extensively by Beaudoin and his coworkers (Cooper, Gupta, and Beaudoin 2000; Cooper et al. 2000, 2002; Cooper, Gupta, and Beaudoin
下一篇: 电解金属沉积物的粘附