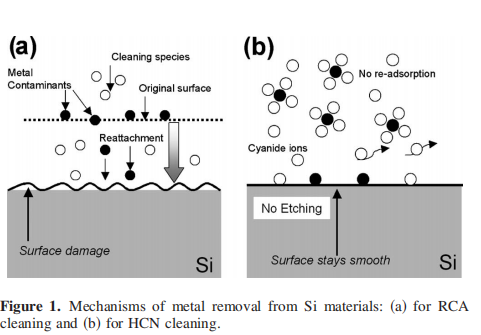
Metal contaminants, such as Cu on SiC surfaces, cannot be completely removed by use of the conventional RCA cleaning method. After RCA cleaning, no chemical oxide is formed on the SiC surfaces, and this chemical stability is attributable to the incomplete removal of metal contaminants by the RCA method because it removes metal contaminants by oxidation and subsequent etching. Cleaning of metal-contaminated SiC with hydrogen cyanide HCN aqueous solutions followed by the RCA cleaning or vice versa can remove them completely. It is concluded that strongly adsorbed metals and metals in the bottom regions on the rough SiC surfaces cannot be removed by the RCA and HCN methods, respectively. The HCN method can remove strongly adsorbed metals because of the high reactivity of cyanide ions, while metals in the bottom regions cannot be removed because of the necessity of the formation of bulky metal-cyanide complex ions for the removal process. © 2008 The Electrochemical Society. DOI: 10.1149/1.2976213 All rights reserved.
SiC has excellent physical properties, such as high breakdown fifield, high electron drift velocity, and high thermal conductivity. Therefore, its application to power devices,1,2 high-frequency devices,3 and devices operated at high temperatures,4 is expected. Cleaning of semiconductors is one of the most important processes for device fabrication, e.g., in the case of fabrication of Si largescale integration LSI , more than 25% of the total processes are for cleaning . Semiconductor cleaning employed in the LSI fabrication processes is usually performed using the RCA method5 i.e., cleaning with a mixture of an ammonia aqueous solution plus a hydrogen peroxide aqueous solution APM , followed by a mixture of a hydrochloric acid solution plus a hydrogen peroxide solution HPM , which mainly remove particle and metal contaminants, respectively. Both the RCA cleaning solutions slightly oxidize and etch Si surfaces, and simultaneously contaminants are removed cf. Fig. 1a . This etching makes the LSI fabrication process complicated. Etching may produce rough surfaces and defect states, which degrade the device characteristics. SiC is much more chemically stable than Si e.g., oxidation of SiC requires heating above 1000°C,6-8 which is more than 200°C higher than the Si oxidation temperature . 9 Although the conventional cleaning method used for Si devices may not be adoptable to SiC due to its high chemical stability and detailed research regarding the mechanisms that are involved during cleaning is lacking, the RCA method is often presumed to be the only suitable technique for SiC cleaning.10-12 Mechanical polishing techniques seem to be the main surfacesmoothing treatments, which may lead to additional metal contaminants on the surface. Chemical mechanical polishing CMP scratch marks are often visible on research grade SiC wafers. Because of this lack of high-quality surface treatment, including cleaning methods in addition to crystal imperfections such as micropipes, dislocation, and crystal grain boundaries, SiC fabrication fails to meet its potential desirable goals.
We have recently developed a method of removal of metal contaminants from Si materials by use of cyanide solutions.13-16 Cyanide ions CN− in the solutions directly react with metal contaminants, resulting in the formation of metal-cyano complex ions and, thus, in desorption cf. Fig. 1b . Metal removal is not accompanied by etching, and readsorption of the complex ions does not occur because of high stability of metal-cyanide complex ions in the solutions. Moreover, CN− ions are selectively adsorbed on defect states, such as Si dangling bonds,17-20 leading to the formation of Si–CN bonds and, thus, to the defect passivation at the same time as metal removal takes place.
In the present study, the mechanism of the RCA method, especially the HPM technique, has been investigated, and it has been shown that metal contaminants on SiC can be completely removed only in cases where two kinds of cleaning solutions i.e., HPM and hydrogen cyanide HCN solutions are successively used.
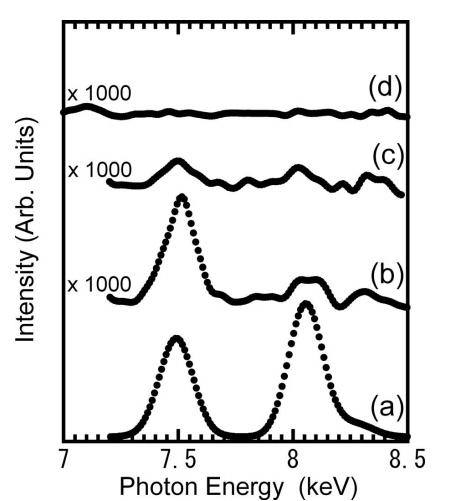
Experimental First, 4H–SiC 0001 wafers were cleaned using the RCA method. Then, the wafers were immersed in 0.08 M CuCl2 plus 0.08 M NiCl2 solutions with a pH of 9 at room temperature for 10 min for intensive contamination, followed by rinse with ultrapure water for 10 min. Then, the SiC wafers were cleaned using APM 29 wt % NH4OH: 30 wt % H2O2: H2O = 1:1:5 , HPM 36 wt % HCl: 30 wt % H2O2: H2O = 1:1:5 , or 0.05 M i.e., 0.14 wt % HCN aqueous solutions. It should be noted that the HCN concentration was much lower than those of the APM and HPM solutions. Cleaning with APM and HPM was carried out at 50 and 80°C, respectively, whereas HCN cleaning was performed at room temperature. The pH of the HCN aqueous solutions was adjusted at 10 by addition of ammonia aqueous solutions. During cleaning, no intensive stirring was carried out. Concentrations of Cu and Ni on the SiC surfaces were determined from total reflflection X-ray flfluorescence TXRF measurements using a Technos TREX 610 spectrometer. The incident angle with respect to the surface-parallel direction was set at 0.05 °. X-ray photoelectron spectroscopy XPS measurements were performed using a VG Scientifific Escalab 220i-XL spectrometer with a monochromatic Al K radiation source. Photoelectrons were collected in the surface-normal direction. Atomic force micrography AFM measurements were carried out with the tapping mode using a Keyence VN-8010 apparatus.
上一篇: 实现新的硅表面处理标准的湿法清洁方法
下一篇: 外延晶圆中金属污染的表征