Abstract:
Hydrogen termination of oxidized silicon in hydrofluoric acid results from an etching processthat is now well understood and accepted. This surface has become a standard for studies of surfacescience and an important component in silicon device processing for microelectronics, energy, and sensorapplications. The present work shows that HF etching of oxidized silicon carbide (SiC) leads to a verydifferent surface termination, whether the surface is carbon or silicon terminated. Specifically, the siliconcarbide surfaces are hydrophilic with hydroxyl termination, resulting from the inability of HF to remove thelast oxygen layer at the oxide/SiC interface. The final surface chemistry and stability critically depend onthe crystal face and surface stoichiometry. These surface properties affect the ability to chemicallyfunctionalize the surface and therefore impact how SiC can be used for biomedical applications.
l.Introduction
Silicon dioxide (SiO,) is the single most versatile materialin technology.Applications extend from optical fibers, transpar-ent structures, and biosensor structures to its critical role inmicroelectronics owing to its amazingly good interface with Si.The latter is the critical property that has enabled all of moderncomputing and communications. Consequently, much is knownabout its chemistry, and broad general knowledge has beenestablished. One such accepted process is that SiO, is dissolvedin HF acid, leaving a hydrophobic H-terminated Si surfacel.2that is almost devoid of surface states. The dangling bonds arefully terminated,3 and this surface displays a high degree ofinertness to further room temperature chemical reactivity.4.5 Thishydrogen-terminated surface has become a “standard’ for manysurface science studies. It is of great interest to understand whyother group IV compounds, including those with a Si atomicsurface, (e.g, silicon carbide) behave differently.
Silicon carbide (SiC) is beginning to replace silicon for highpower, high-temperature applications and is being consideredfor high- and midvoltage MOSFET devices. For electronicapplications, the attraction of SiC stems from the ability to growa thermal silicon dioxide passivating layer, similar to siliconwhich suggests that the wet chemical cleaning methods (e.g..HF etching) that have been well developed for Si can be usedfor SiC. Another important attraction of silicon carbide comesfrom its stability and biocompatibility, important for biomedicaluses, such as a material for implants (in bone tissues), stents inblood vessels, and substrate for biosensors. For these applica-tions, understanding and controlling the surface termination ofits surface is critical. In particular, performing HF etching is animportant step that is common for these applications.
Stoichiometric SiO, can be thermally grown on both Si andSiC, and no major difference is expected with regard to bulkoxide etching by HF, as has been noted previously.° HF etchingof the bulk oxide proceeds by H,O and SiF, removal.7 The finalsurface termination is well understood in the case of siliconAs the last oxygen atoms are removed from the silicon surfacethe topmost silicon layer is F terminated. As the reactioncontinues however the Si back bonds are polarized, making themsusceptible to further attack by HF to remove the top fluorinatedSi atom and leaving the second layer Si atom with H termina-tion, as shown schematically in Figure la. The situation is quitedifferent for SiC surfaces on both the Si-terminated and C-terminated surfaces, as schematically shown in Figure 1b and discussed below.
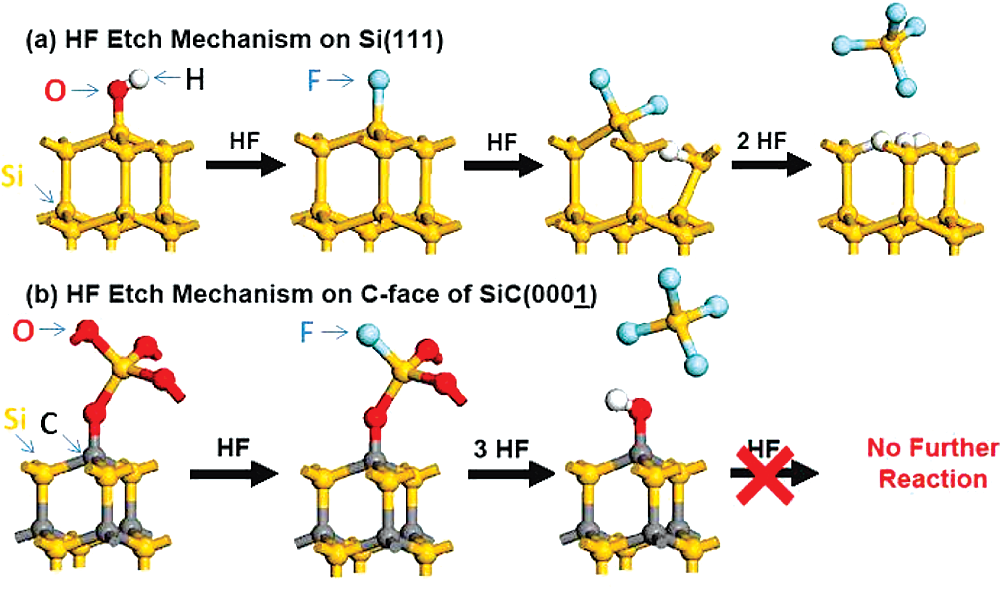
Figure 1
II. Background Differences between Si/SiO2 and SiC/SiO2 interfaces have been noted in earlier works.8-13 Using ex-situ X-ray photoelectron spectroscopy (XPS), Sieber et al. reported that oxygen was present on the Si-face of 6H-SiC following HF treatment, although postcontamination in air could not be ruled out.11 Seyller showed different low-energy electron diffraction (LEED) patterns between wet-cleaned and hydrogen-annealed 6H-SiC (0001) surfaces,10 suggesting that HF-etched SiC are not simply H terminated. Using vibrational spectroscopy after HF treatment of both (0001) Si- and (0001j) C-terminated faces of 6H-SiC, Tsuchida et al. reported that surface-bound H (C-Hx, Si-H) was not detectable on either surface and instead presented evidence for the presence of some hydroxyl (OH) groups after HF treatment, possibly due to contamination in air.13 Surface wetting of SiC was also found to be different compared to Si surfaces.8,14,15 In particular, King et al. examined the wetting characteristics by immersion in a variety of acid/base solutions and found SiC surfaces to be mostly hydrophilic.8 More recently, evidence for oxygen on the surface was interpreted as due to the formation of a silicon oxycarbide (SiOC) alloy at the surface, associated with the initial thermal oxidation.
上一篇: 硅在HF溶液中的阳极溶解