Experimental
Materials used for the etching studies were Fe-doped InP and Cr-doped GaAs substrates, and GaInP grown on GaAs substrates by organometallic vapor phase epitaxy (OMVPE). The GaInP layers were 500- nm thick, silicon doped (n = 1 x 1017 cm-3), and nearly lattice-matched to GaAs (nominal composition of Ga0.~2In0.48P. The samples were patterned photolithographically with 100 ~m squares using AZ5214 photoresist in image-reversal mode. When adequately hard-baked, the resist served as an etch mask and resisted attack well in most etchants. Etchant solutions were prepared by mixing standard solutions of HC1 [36 weight percent (w/o)], H2Q (30 w/o), and glacial acetic acid (CH3COOH 99.9%) in various proportions. Typically, the HC1 and CH~COOH were premixed, and the H2Q was added seconds before emersing the samples in the solution. The samples were typically etched at 20~ for 1 min without stirring or agitation, and were subsequently quenched in running deionized water and blown dry with nitrogen. The etch depth was measured using a Sloan Dektak profilometer, and the smoothness of the etched surface was evaluated by optical and scanning electron microscopy.
Results and Discussion
The effect of an oxidizing agent on the etching characteristics was examined by varying the amount of H202 in a mixture of 1 HCI:20 CH3COOH:xH2Q, with 0 -< x --< 5. The etch rates in Fig. 1 represent the first minute after H2Q is added to the mixture. When no oxidizing agent is present, both GaInP and InP are etched selectively with respect to GaAs. The etch rates are approximately 4000, 700, and less than 50 A/min for InR GaInP, and GaAs, respectively.
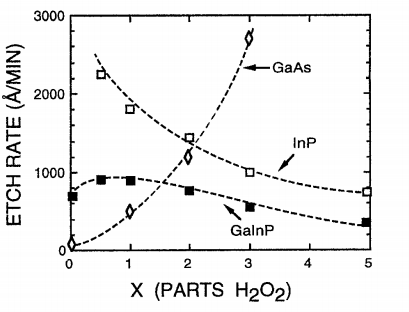
Fig1
To evaluate further the usefulness of these etchants for a gate recess process, the stability of a mixture of 1 HCI:40 CH3COOH:I H2Q was evaluated by measuring the change in etch rate with the age of the solution. Fig. 5 shows that the etch rate for all materials increases strongly over the first 40 rain after mixing, levels out, and then slowly decays. Initially, the relative etch rates vary as InP > GaInP > GaAs. However, within 20 rain all materials etch at comparable rates s,howing only slight selectivity with relative etch rates GaAs > InP > GaInP. The surface morphology remained relatively smooth with increasing age of solution for all three materials.
The change in the relative etch rates suggests that a change in the dominant reaction mechanism occurs over time. One can gain insight into the changing chemical mechanisms of this system by considering the thermodynamics of chloride oxidation by H202. In aqueous solutions Cl- is oxidized by H~O2, and the reactions listed in Table I can occur. The standard Gibb's free energy change, AG ~ for each reaction has been calculated from standard electrochemical potentials. 13 When considering the value of AG~ mole of H202 consumed, the reaction producing Cl2(g) is the most favorable. Other reactions which result in chemical species containing chlorine in higher oxidation states are also highly exothermic.
An idea] contact does not exhibit any interfaces. In fact, practical contacts differ from that ideal behavior mainly due to (i) differences in the work functions of metal and semiconductor yielding potential barriers, (it) surface states of the semiconductor, and (iii) poor adhesion.
上一篇: 氮化镓的深紫外增强湿法化学蚀刻
下一篇: InGaP和GaAs在HCl中的湿蚀刻