Cu has been widely accepted as an interconnection material in deep submicrometer multilevel device applications because of its lower resistance, superior resistance to electromigration, and the reduction of resistance-capacitance ~ RC! time delay compared with aluminum.1 The Cu interconnection is made possible by novel damascene chemical mechanical planarization ~ CMP! . During Cu CMP, wafer surfaces are exposed to at least two different slurry solutions. After polishing, the removal of abrasive particles and trace metals left on the wafer surfaces becomes as important as the polishing process itself in order to maintain device yield.2 Since the CMP process leaves these contaminants on the wafer surface, post-Cu CMP cleaning is a necessary step to eliminate or reduce them before the next process step. Earlier studies reported on the effect of pH on removal and adhesion of silica particles in slurry solutions,3 however, very little has been reported on the effects of additives in cleaning solutions on removal and adhesion of particles of interest to post-Cu CMP cleaning.
The adhesion force of particles on Cu surfaces was measured using an AFM ~ Park Scientifific Instruments CP Research! by directly measuring the force required to remove them from a surface.5,6 A 40 m m diam sized spherical silica particle ~ Duke Scientifific, Co.! was attached on a Si3N4 tipless cantilever ~ Fig. 1! . The adhesion force was measured between the particle and the wafer surface in a liquid cell. Electroplated Cu wafers ~ 10,000 Å, 0.016 V /h ! were used for the experiments. They were precleaned in dilute HF ~ DHF, 0.01 vol %! solution for 30 s before the measurements. The post-Cu CMP cleaning solution used included citric acid, corrosion inhibitor, and pH adjustors. BTA ~ benzotriazole! was used as a corrosion inhibitor. NH4OH and TMAH ~ tetramethylammonium hydroxide! were used to adjust the pH.
In order to investigate the surface adsorption of citric acid on silica particle, the zeta potential of silica particles was measured as a function of pH with and without the addition of 1,000 ppm of citric acid in 102 3 M KCl solutions as shown in Fig. 2a. Citric acid gives silica particles more negative zeta potential due to the adsorption of citrates on silica surfaces. The presence of citric acid resulted in slightly more negative zeta potential than values observed in silica particles at the same pH. Figure 2b shows the zeta potential of Cu particles as a function of pH with and without the addition of citric acid. BTA was added to prevent Cu dissolution at acidic pH. Slightly more negative zeta potentials were also observed on Cu surfaces with the presence of citric acid.
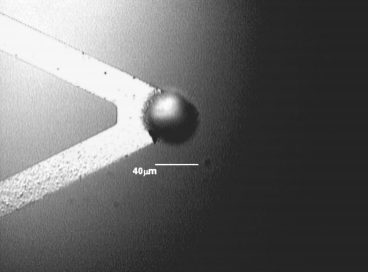
Fig1
The interaction force between particles and surfaces can be measured using AFM as the attached particle on the cantilever approaches the substrate.8,9 Forces between the attached particle and the sample surface cause the cantilever to bend or deflflect. A detector measured the cantilever deflflection as the particle approaches the surface of the sample. From the force-distance curve, the magnitude of interaction force between the two surfaces could be evaluated. Figure 3a shows the measured adhesion forces between the spherical silica particle and Cu surfaces. The adhesion force of a silica particle on Cu surfaces decreased from 2.0 to 0.2 nN as the concentration of citric acid increased to 0.7 wt %, and then reached a constant value. Even though there was no change in pH ~; 2! in the concentration ranges investigated, the decrease of the adhesion force may be attributed to the adsorption of citrate ions on Cu and silica, resulting in more negative and repulsive zeta potentials on these surfaces as shown in Fig. 3b. The increase of magnitude of zeta potentials on surfaces caused a stronger repulsion of particles from surface.
上一篇: CMP后晶片表面金属污染物的清洗方法
下一篇: 光刻烘烤过程中的晶圆翘曲检测