Abstract: We investigate the interest of deep wet etching with HF/HNO3 or KOH solutions as a final step after polishing to improve fused silica optics laser damage resistance at the wavelength of 351 nm. This comparison is carried out on scratches engineered on high damage threshold polished fused silica optics. We evidence that both KOH and HF/HNO3 solutions are efficient to passivate scratches and thus improve their damage threshold up to the level of the polished surface. The effect of these wet etchings on surface roughness and aspect is also studied. We show that KOH solution exhibit better overall surface quality that HF/HNO3 solution in the tested conditions. Given the safety difficulties associated with the processing with HF, KOH solution appears as a pertinent alternative to HF deep wet etching. © 2017 Optical Society of America.
High power Inertial Confinement Fusion (ICF) laser systems, such as the National Ignition Facility (NIF, USA), the Laser MegaJoule (LMJ, France) and the SG series Laser Facility (SG-III, China), use fused silica optics as final optics operating at 351 nm. The life time of these optical components is reduced by laser damage which occurs under UV illumination and fluence in the 5-20 J/cm2 range for nanosecond pulse duration.
Pollutants from the polishing fluid and defects such as surface scratches, digs and subsurface damage (SSD) are main precursors of laser damage. Experimental studies explained phenomena which are responsible of subsurface and surface damage creationand impurities penetration during the polishing process. Numerical studies completed the experimental works to understand and predict the influence of these precursors on the laser induced damage threshold (LIDT). For example, Gao et al. proposed a thermal model based on the heating of inclusions to a critical temperature in order to evaluate the influence of various impurities on the LIDT. Researches about the influence of scratches and SSD revealed that these defects reduce the laser damage resistance because of electromagnetic field intensification and enhanced absorption due to surface features and possible local absorbers. With these highlights, the polishing process has been optimized to reduce the amount of surface and subsurface defects. For example, investigations about the magnetorheological finishing (MRF) provided evidence that this polishing technique was able to remove surface and subsurface defects and so improved LIDT of fused silica optics operating in UV light when combined with a small wet chemical etching [15,16]. At this step, laser damage performance of surface was considerably improved up to the level needed for ICF laser facilities and became limited mainly by surface defects such as scratches and digs which thus had to be passivated.
Optics for the NIF and the SG series Laser facilities are usually treated with a solution composed of HF and NH4F which is named Buffered Oxyde Etch (BOE). With this chemical solution, the ammonium group (NH4) is responsible for the generation of (NH4)2SiF6 precipitates [19] and can also diffuse in silica reacting with structural defects as Frenkel defects and dangling bond defects [25]. Unfortunately, these parasitical phenomena could limit the LIDT enhancement because the ammonium group could absorb the laser energy and trigger laser damage. Consequently, the BOE etching needs to be optimized by the addition of an ultrasonic agitation. This agitation is not necessary for an etching with a solution composed of HF and HNO3 or HF only.
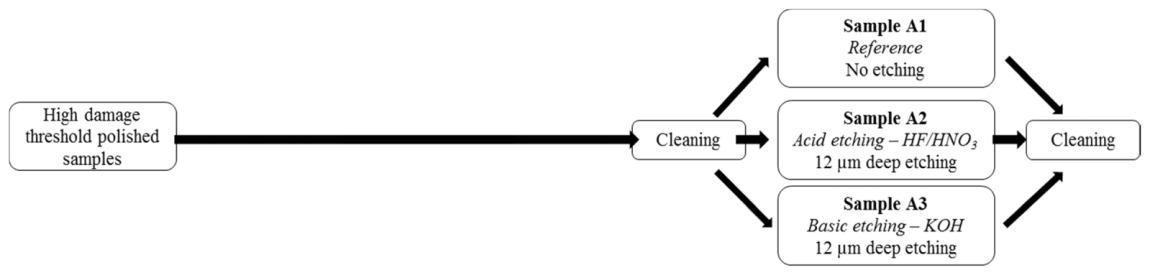
Fig1
The first batch (named samples A1 to A3) is reserved for polished surface characterization whereas the second batch (named samples B1 to B3) concerns the characterization of scratches. So the second batch needed intermediate steps to make the scratches and to show them up. Scratches were created by polishing using a single side polishing machine (Logitech PM5). Polishing was 30 min long and the slurry was composed of cerium oxide particles in colloidal silica. To remove the polishing layer and reveal scratches, a light wet etching (2 µm deep) was performed on these samples with a mixture of hydrofluoric acid HF (2.7wt%) and nitric acid HNO3 (22.8wt%) at room temperature and with the system presented in Fig. 3. The two batches of samples were cleaned in an automatic washing machine. The cycle started with a washing using a basic detergent at 60°C, then a rinsing, a second washing using an acid detergent at 40°C and finished with several rinsing steps.