Since the discovery of the role of oxidative etching in shape-controlled metal nanostructure synthesis in 2004, it has become a versatile tool to precisely manipulate the nucleation and growth of metal nanocrystals at the atomic level. Subsequent research has shown that oxidative etching can be used to reshape nanocrystals via atomic addition and subtraction. This research has attracted extensive attention from the community because of its promising practical applications and theoretical value, and as a result, tremendous efffforts from numerous research groups have been made to expand and apply this method to their own research. In this review, we first outline the merits of oxidative etching for the controlled synthesis of metal nanocrystals. We then summarize recent progress in the use of oxidative etching to control the morphology of a nanostructure during and after its synthesis, and analyze its specific functions in controlling a variety of nanocrystal parameters. Applications enabled by oxidative etching are also briefly presented to show its practical impact. Finally, we discuss the challenges and opportunities for further development of oxidative etching in nanocrystals synthesis.
In the past decade, a series of metal nanocrystals with various geometries have been synthesized by adjusting a number of experimental parameters including the metal precursor, solvent, temperature, the catalyst, the capping agent, and the reducing agent.1,2,29–45 Each of these parameters can have an impact on the nucleation and growth of metal nanocrystals, providing the means for controlling the crystallinity and surface facets of nanocrystals.1,46 Despite the success in precisely tailoring nanocrystal parameters, many questions regarding how to improve reaction reproducibility and product homogeneity remain unanswered. Indeed, these questions have puzzled the research community since shape-controlled synthesis was first developed (i.e. late 1990’s and early 2000’s).1 Addressing these questions requires a deeper understanding of the fundamentals behind each synthesis.
In a typical synthesis, the formation of metal nanocrystals can be divided into three stages: nucleation from the atoms formed by reduction of metal salts, evolution of nuclei into seeds, and growth of seeds into nanocrystals through a process of atomic addition.1 Tremendous efffforts have been made to manipulate the way the atoms are added to a seed. For example, nanocrystal growth rates can be manipulated by altering the reducing agent, temperature, solvent, and the capping agent. Basically there are two important aspects of the atomic addition process: one is the reduction rate of ions to zerovalent atoms, and the other is the location where atoms are added to seeds. Once atoms are added to specific locations on the formed seeds, they will act as a template to guide further atomic addition. Without a mechanism for reversing atomic addition, metal nanocrystals can continue to grow to form morphologies that are thermodynamically unfavorable.
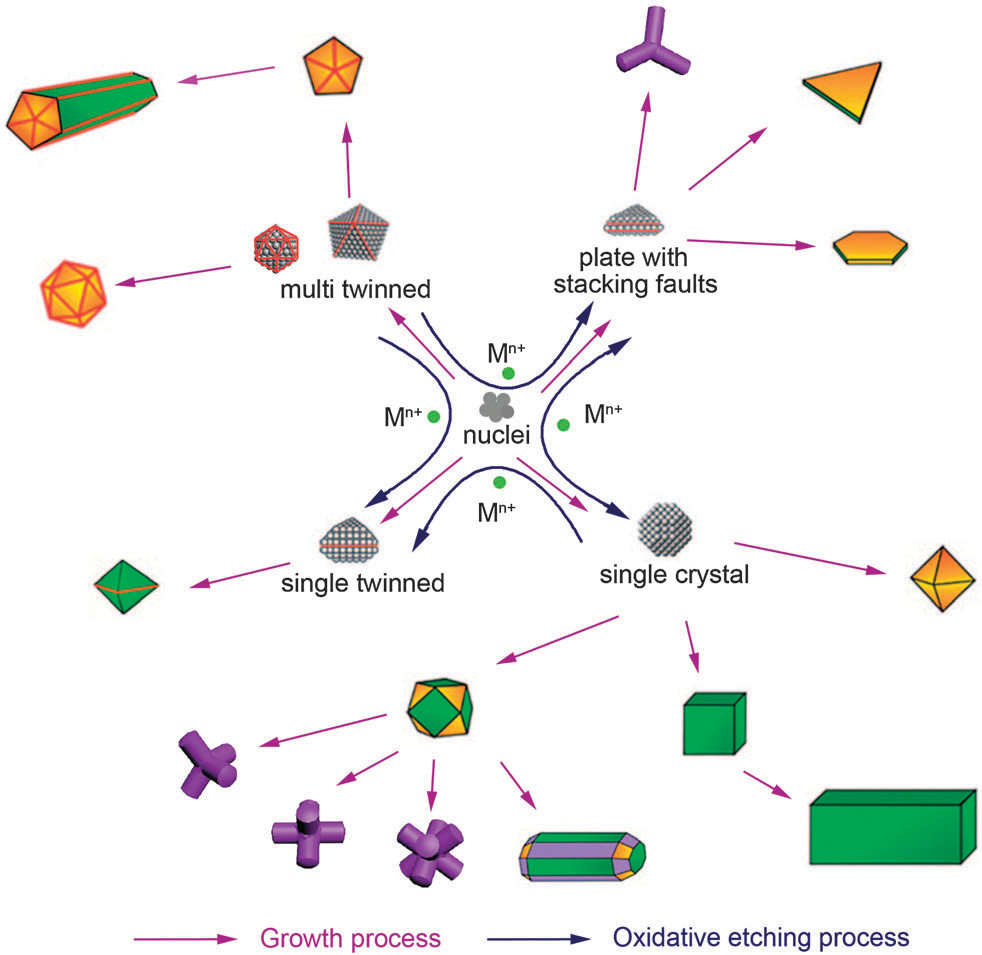
Fig1
In this review article, we focus on the recently developed strategies for the synthesis of metal nanocrystals that used oxidative etching, and highlight the common mechanisms in various synthetic systems and approaches. Given that metal elements account for 2/3 of the periodic table, we will narrow our discussion down to the system of fcc metals. In the first part of this review, we describe the nature of oxidative etching in order to understand its effffect on the synthesis of nanoscale metal materials. We then discuss a series of important control factors enabled by oxidative etching. We finish the synthetic discussion by showing how oxidative etching can be employed as a post-synthesis tool for atomic addition and subtraction to maneuver the size, structure and shape of metal nanocrystals. In the last section of this review, we highlight a few applications enabled by catalytic and optical properties tuned with oxidative etching