Thin-film yield in the chemical bath deposition technique is studied as a function of separation between substrates in batch production. Based on a mathematical model, it is proposed and experimentally verified in the case of CdS thin films that the film thickness reaches an asymptotic maximum with increase in substrate separation. It is shown that at a separation less than 1 mm between substrates the yield, i.e. percentage in moles of a soluble cadmium salt deposited as a thin film of CdS, can exceed 50%. This behaviour is explained on the basis of the existence of a critical layer of solution near the substrate, within which the relevant ionic species have a higher probability of interacting with the thin-film layer than of contributing to precipitate formation. The critical layer depends on the solution composition and the temperature of the bath as well as the duration of deposition. An effective value for the critical layer thickness has been defined as half the substrate separation at which 90% of the maximum film thickness for the particular bath composition, bath temperature and duration of deposition is obtained. In the case of CdS thin films studied as an example, the critical layer is found to extend from 0.5 to 2.5 mm from the substrate surface, depending on the deposition conditions.
Chemical bath deposition is a thin-film technique in which compound semiconductor thin films of typically 0.02–1 µm thickness are deposited on substrates placed in contact with dilute baths containing metal ions and a source of sulphide or selenide ions. Many I–VI, II–VI, IV–VI and V–VI semiconductors are included in the list of materials deposited by this technique. In some cases metal hydroxide thin films are deposited and subsequently converted into an oxide film. Recently, thin-film formation by this technique has been studied at a more fundamental level. In a phenomenological model for the deposition of semiconductor thin films by chemical bath deposition, we have suggested an overall equation to describe the kinetics of thin-film deposition [6]. This model took into account the following aspects, that are summarized in table 1.
We would like to mention here that in this model, summarized in table 1, we have assumed an overly simplified chemistry of the deposition process. In chemical bath deposition the concentration of each of the components in the deposition bath, and not just the concentration of metal ions, plays an important role in deciding the growth rate and film thickness, as discussed in the case of CdS thin films. We consider that eventually a model containing the deposition parameters discussed in this paper would be combined with the detailed chemistry of the deposition process to make it fundamentally sound. The physico-chemical meaning of the parameters given in table 1, which have been proposed as fitting parameters in this paper and in [6], would emerge in subsequent investigation.
The purpose of this paper is to establish the dependence of the thin-film process yield on the separation between substrates during the deposition. We shall use the case of chemically deposited CdS thin film as an example and establish that there exists an optimum separation between substrates at which, for a given bath composition and temperature, 90% of the maximum possible process yield is achieved. Substrate separation larger than this value contributes only marginal benefit to film thickness, but leads to a considerable drop in the process yield. We consider the discussion in this paper will contribute towards furthering technological prospects for chemically deposited semiconductor thin films.
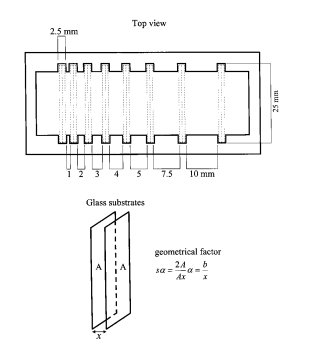
Fig1
We reported earlier that CdS thin films can be deposited from the bath constituted as above at 50–90 ◦C. In this case, we chose the following conditions: duration of depositions of 8 and 24 h at 60 ℃ and of 8 h at 75 ℃. In the case of deposition at 60 ℃ for 8 h, the terminal thickness is not expected. The experiment was performed to check whether the presence of a higher volume of the deposition bath would essentially contribute to a higher initial rate of deposition. At the end of the deposition the substrates were taken out, washed in distilled/deionized water and dried. As the substrates were held in pairs by sticking together with a water drop, the thin-film coatings were obtained on each substrate on the side that faced the solution. The inner side of each pair of substrates was cleaned to remove traces of deposit by cotton swabs moistened in dilute HCl.
上一篇: 半导体晶片光调制热反射率的两层模型
下一篇: 快速热处理后硅晶片的精密化学分析开发