Second-order nonlinear optical (NLO) crystals functioning in the ultraviolet (UV) and deep-ultraviolet (deep-UV) regions are critically important as frequency conversion materials for all-solid-state UV laser devices. In this review, we focus on recent studies of inorganic UV and deep-UV NLO crystals with wide UV transparency and SHG efficiency. Following an introduction describing crystal-design strategies for UV and deep-UV NLO materials, the manuscript is organized according to the type of inorganic anions (borates, carbonates, nitrates and phosphates). Special attention is given to the crystal structures, second-order NLO properties, and structure−property correlations. The concluding remarks highlight future prospects in the field, with emphasis on the superiority of the different types of UV and deep-UV NLO crystals and the importance of large-size crystal growth for practical application in electro-optic devices.
NLO-active crystals that can be used in the UV and deep-UV regions require a large transparency window (i.e. a wide bandgap), large SHG coefficients, moderate birefringence, chemical stability and facile growth of large single crystals . The planar [BO3] 3- and isoelectronic anions such as [NO3] - and [CO3] 2- , which possess moderate birefringence and large microscopic second-order hyperpolarizabilities β, are believed to be the most favorable anionic units for UV and deep-UV candidates. Phosphates have attracted attention as potential UV and deep-UV NLO candidates in recent years due to their wide UV transparency and the ease with which one can grow bulk crystals . As far as the metal cations are concerned, alkali and alkaline-earth cations with no d−d or f−f electronic transitions and rare-earth cations with fullyoccupied d (3d 10) or half-occupied f (4f 7 ) electronic shells are commonly used in the pursuit of UV and deep-UV NLO crystals . The introduction of halogens has been shown to influence crystal structure and cause a blue shift in the UV absorption spectra .
This review highlights recent progress in the construction of second-order NLO inorganic crystals that are active in the UV and deep-UV regions, with a particular focus on their crystal structures, SHG properties, and structure-property relationships. Well-known NLO-active crystals such as β-BaB2O4 (BBO) , LiB3O5 (LBO) , CsLiB6O10 (CLBO) , CsB3O5 (CBO) , Sr2Be2B2O7 (SBBO) , and KBe2BO3F2 (KBBF) have been extensively studied and commercialized, so they are not the focus of this review . For the convenience of discussion, NLO crystals functioning in the UV and deep-UV regions are classified into four categories according to the anions: (1) metal borates, (2) metal carbonates, (3) metal nitrates, and (4) metal phosphates. We conclude the review by identifying the current critical challenges that impede the progress of the different types of UV and deep-UV NLO-active crystals into practical applications.
Combinations of [BO3] planar triangles and [BO4] tetrahedra give rise to a diverse array of borate structures. These compounds possess several interesting features. The large difference in electronegativity of the boron and oxygen atoms favors transmission of short-wavelength light, while the conjugated π-orbitals and highly anisotropic electron distribution in the [BO3] groups is beneficial for the generation of large microscopic second-order susceptibilities and birefringence . It has also been noted that elimination of dangling bonds at the oxygens in the B−O groups can effectively increase the energy bandgap and blue-shift the absorption edge of borate crystals . SHG-active metal borates can be classified into four categories based on their chemical composition: metal borates without additional anions; metal borates containing halides; metal hydrated borates; metal borates with mixed anions.
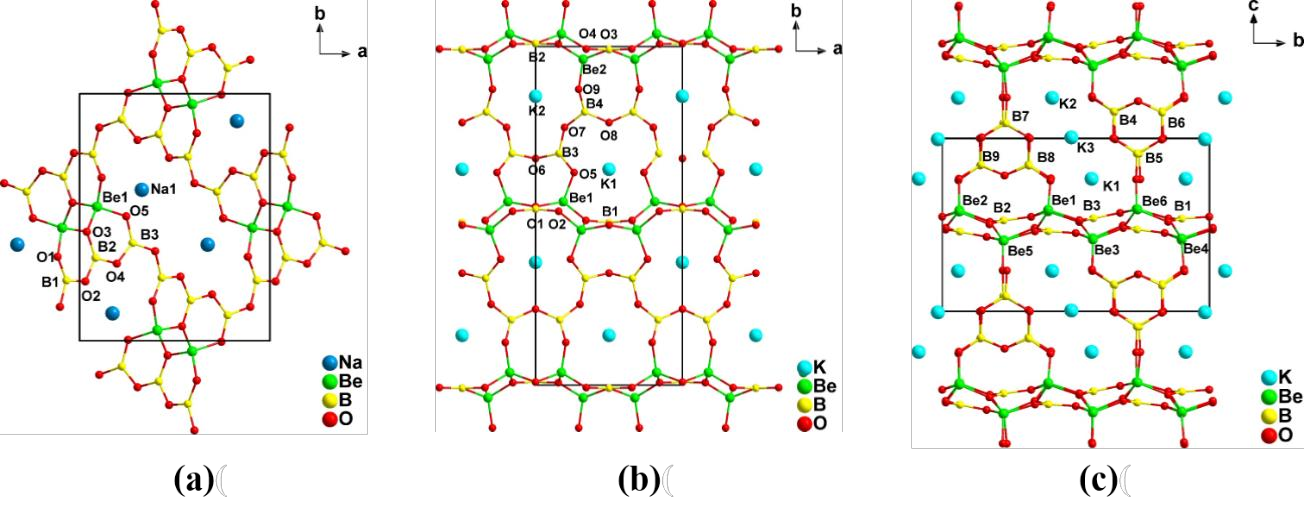
Fig1
Metal borates without additional anions are usually prepared by solid-state reaction techniques and, thus far, several KBBF-family crystals with improved layer growth have been reported. However, these crystals do not possess the optical advantages of KBBF, while their toxic Be content decreases their applicability. A few crystals exploiting nontoxic elements have been reported (e.g. Cs3Zn6B9O21, Li4Sr(BO3)2, β-Rb2Al2B2O7), but large optical-quality crystals are needed.
The hydrothermal method, solvothermal method and solution evaporation method are the conventional routes to synthesize hydrated borates. Varying the reaction conditions (e.g. modifying the reaction temperature, counterion, concentration of boron in the solution, and the pH value of the reaction media) can afford metal borates with a range of structures, including discrete B−O clusters, infinite chains, sheets, and 3D frameworks. In the process, many NCS crystals with excellent SHG properties have been discovered (Table 3).
上一篇: 直接晶圆键合中空隙的光声成像
下一篇: 紫外激光去除硅晶片中的小金属颗粒