Thin-film solar cells based on polycrystalline p-type Cu(In1-xGax)Se2 absorbers show the highest power conversion effificiencies (>21%)1 among all thin-film solar cell photovoltaic materials. Typical chalcopyrite solar cells consist of a stack of various semiconductors and metals, consecutively deposited onto glass substrates. In this multilayer architecture, the chemical, structural, and electronic interface properties play a crucial role for the overall performance of the final solar cell device. In this respect, with the goal to improve understanding of the interface formation and properties, and to ultimately improve solar cell efficiencies, a significant number of surface and interface studies have been performed in the past, many of those using photoemission spectroscopy2–9 or scanning probe microscopy.10–14 The conclusions based on the findings from these techniques mainly rely on the measurement of clean sample surfaces under ultrahigh vacuum (UHV) conditions. However, for such kind of studies, samples are typically obtained from independent growth equipment and are transferred through air into the UHV of the experimental setup for characterization. Contamination and oxidation can signficantly inflfluence the results of these characterization techniques. Therefore, it is important to create reproducible and well defifined surfaces with respect to their composition that are free of contamination.
In the past, results on the oxidation of Cu(In1-xGax)Se2 surfaces and their chemical etching have been presented. It was found that etching in NH3- and KCN-based solutions can remove undesired secondary phases and oxides from the surface of Cu(In,Ga)(S,Se)2, as, i.e., Cu-selenides or SeO2. Kazmerski et al. studied the oxidation of CuInSe2 by controlled oxygen exposure under UHV conditions and the subsequent cleaning using ion etching.15 An oxidation study of CuGaSe2 was presented by W€urz et al., 6 who compared the oxidation after different exposure times to air. X-ray photoelectron spectroscopy (XPS) was then used to analyze the different oxide phases present at the surface. However, so far, a systematic study of how good wetchemical surface cleaning procedures work for different degrees of surface oxidation is still missing.
In this paper, we present a study of the native oxidation of polycrystalline Cu(In1-xGax)Se2 thin films, which were oxidized for different periods of time and subsequently wet-chemically treated in basic solutions (KCN or NH3). The composition and the degree of oxidation were analyzed using surface sensitive XPS and x-ray excited Auger electron (XAES) spectroscopy. Mg Ka and Al Ka radiation were used as complementary excitation sources to gain depth-resolved information. The results of our study indicate that a KCN etch provides a chemical surface structure almost identical to a pristine sample surface.
Immediately after the defined air exposure, the surface of each sample was wet-chemically treated using KCN- or NH3-based aqueous solutions. The NH3 treatment consisted of dipping the sample in 1.5 mol/l aqueous NH3 solution for 10 s, 100 s, or 1000 s at room temperature. This treatment resembles cleaning processes similar to those occurring during the standard wet-chemical CdS buffer deposition. For the KCN treatment, the CuIn0.71Ga0.29Se2 layers were dipped into 0.15 mol/l aqueous KCN solution for 2 min also at room temperature. After the respective treatments, all samples were rinsed with N2-purged de-ionized water and subsequently dried with N2. The surface treatment of all samples was performed under inert gas atmosphere in a N2-filled glovebox with an O2 concentration of 1 ppm attached to the UHV chamber of the XPS setup. Therefore, post-cleaning re-exposure of the samples to ambient conditions could be minimized.
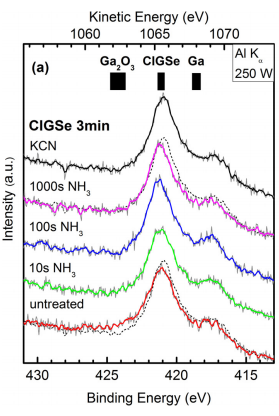
Fig1
Quantitative analysis of the surface composition was obtained from fits of specific photoemission lines of the involved elements. In the following, we present the analysis for each element in the sequence as presented for the qualitative analysis above. The surface content of gallium was analyzed using the Ga 2p3/2 photoemission line (see Fig. 4). As a result of the already discussed presence of Ga2O3 at the CuIn0.71Ga0.29Se2 surface (see above), an asymmetric broadening of the peak towards higher binding energies is expected and can be observed—least prominent for the untreated samples and increasing with air exposure time. Hence, the Ga 2p3/2 line was fitted by two Voigt functions. Fig. 4 shows the experimental data and the fits of the Ga 2p3/2 line as a function of the samples exposure time to air and chemical surface treatment.
Next, we address the quantification of the formation of surface indium oxide on the air-exposed CuIn0.71Ga0.29Se2 surface. The corresponding In 3d3/2 photoemission lines are shown for all air-exposure times and wet-chemical treatments in Fig. 5. Similarly to the Ga 2p3/2 photoemission line, also for the In 3d3/2 peak, we find an increased asymmetry towards higher binding energies with increased air exposure time. Hence, also the In 3d3/2 line was fitted with two Voigt functions. The main contribution was found to be at EB ¼ (452.2 6 0.1) eV and attributed to indium in a chalcopyrite environment. The component at higher binding energy EB ¼ (453.0 6 0.1) eV was ascribed to In2O3. Both, the derived binding energy positions as well as the binding energy difference DE ¼ 0.8 eV are in agreement with literature values for chalcopyrites and In2O3. 15 The derived increasing In2O3 contribution to the In 3d3/2 photoemission line with increasing air exposure time confirms the above formulated observation of a more pronounced peak asymmetry. Again in agreement with the more qualitative assessment of the surface indium oxidation based on the In M45N45N45 Auger spectra shown in Fig. 2, the In 3d3/2 XPS data indicate the complete removal of the surface oxide from the “CIGSe 100 h” sample by the 1000 s NH3 and KCN treatment but not—by any treatment—from the “CIGSe 1000 h” sample.
For the XPS/XAES measurements, Mg Ka and Al Ka radiation as excitation sources and a CLAM 4 analyzer (VGScienta) with a 9-channeltron detector were used, operated in an analysis chamber at a base pressure of 10-10 mbar. The calibration of the energy scale was carried out according to reported methods and reference data.19 Characteristic photoemission and Auger lines of Ga, In, Cu, and Se were recorded as a function of the sample’s air exposure time and surface treatment for further qualitative data analysis.
上一篇: 损失最小的超薄等离子硅晶片太阳能电池
下一篇: 金属沉积过程中晶圆制造的颗粒减少