In recent years, there has been a noticeableincrease in collecting and analyzing large amounts ofdata from process equipment. The utilization of this datato detect abnormalities at an early stage and to improvequality of the processes has been enabled by the rapidadvancement of data storage and analysis capabilities.Thanks to the evolution of semiconductor hardware andsoftware, vast amounts of data can be stored andprocessed. Access to data and analysis that affects thequality of processed wafers is important for the smart fabconcept.
The current main application of the quadrupolemass spectrometer (QMS) in vacuum processes isresidual gases monitoring, we refer to this data as“Condition monitoring”. Figure 1 shows the trend ofresidual gases during vacuuming after chambermaintenance. H2O is readily absorbed to the chamberinner wall. By applying a baking process during the initialpump down of the process chamber, residual H2O partialpressure can be reduced by an order of magnitude.Users can set a threshold pressure at which theproduction process can be started, or multiple gas datacan be used to develop an algorithm to determine it.
Another QMS application is “Process monitoring”that enables the real-time observation of changes in gasspecies in a chamber during wafer processing. There aremany process parameters, e.g. gas flow rates, thatdetermine the quality of processes, most of which arecontrolled precisely through process recipes. When itcomes to direct monitoring the inside of a chamber, onlyoptical emission spectroscopy (OES) is used for plasmaprocesses in mass production. Although OES has beenan essential technology for plasma diagnostic, the QMShas advantages, such as monitoring during non-emission state in the myriad of complex processes required foradvanced semiconductor manufacturing.
This article reports the results of the application ofthe in-situ QMS to plasma process monitoring. The QMSis attached toa dielectric etching chamber to observechanges in gas species during SiN etching. Theeffectiveness of process monitoring with the QMS isexamined througha series of experiments.
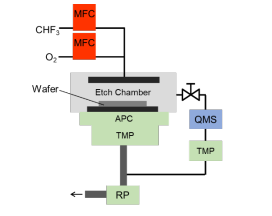
Fig1
A schematic diagram of the experimental setup isshown in Figure3.A QMS (HORIBA MICROPOLEsystem) witha differential pumping system is attached tothe reactive ion etch chamber for plasma measurements.The chamber pressure and the pressure at the QMSduring the etching process are maintained to5 Pa and0.01 Pa, respectively. The electron impact energy in theQMS is set to 70 eV. The CHF3 andO2 flow rates arecontrolled by the mass flow controllers in sucha way thatthe total flow rate is held constant at 30 SCCM. The RFpower at 13.56 MHz is set to 150W. Plasma enhancedchemical vapor deposition-grown SiN layers on Sisubstrate were prepared for this experiment. Etchingrates were confirmed by the ellipsometry (HORIBAAutoSE).
Figure 5 (a) and (b) shows the mass spectraduring the SiN etching for the CHF3/O2 flow rates of 30/0SCCM and 12/18 SCCM, respectively. In the lowestetching condition of CHF3/O2 = 30/0 SCCM (Figure 5 (a)),the main detected ions are CF3+ and CHF2+. Since CHF3gas decomposes into CF3+ and CHF2+ as the first step bythe electron impact in the QMS, namely fragmentation,these ions were observed under both plasma-on andplasma-off conditions. There are oxygen-containing ionpeaks such as CO, even though no oxygen gas isintroduced. This is believed to be due to the residualoxygen-related gases and the exposure of quartz parts tothe etching gas. It is notable that C2F3+ and C3F3+ weredetected for the only plasma-on condition, indicating thatsome fluorocarbon radicals polymerize in the gas phase.