Caria nanoparticles which have been used to remove SiO2 film rapidly and selectively form Ce-O-Sibonds with proposed chemical reaction (-Ce-OH + -Si-O- ↔ Ce-O-Si- + OH-) on the SiO2 film surface whenpolishing swallow trench isolation (STI) and inter layer dielectrics (ILDs). Adsorbed ceria nanoparticles withCe-O-Si bonds are hard to remove from the SiO2 film surface than other residues and harder to removesmaller sized ceria nanoparticles than before.
For the effective removing of residual ceria nanoparticles on the SiO2 film surface, Seo et al. optimizedthe mixture ratio of ammonium hydroxide (30wt% NH4OH) and hydrogen peroxide (30 wt% H2O2) solutionsto maximize the concentrations of perhydroxyl ions (HO2-) as an break the Si-O bond.[2] Further, Gowda etal. developed cleaning solutions with chemical additives as the prohibition for re-adhesion of detached ceriananoparticles from the both SiO2 and Si3N4 film surface.
Moreover, the mixed solution of sulfuric acid (H2SO4) and hydrogen peroxide (H2O2) which called SPMor piranha solution is significantly concerned and used to cleaning process for semiconductor devicemanufacturing due to its effects for not only removing organic residues but also the cleaning of ceriananoparticles with strong oxidizing agent after chemical mechanical planarization (CMP).[4] Especially,single wafer cleaning method using SPM under directly mixing of chemicals in the spray nozzle increasinglyapplied in practical cleaning process.
Even though SPM single cleaning method qualified, study for thermodynamics of SPM solution andcorrelation of cleaning effect is not sufficiently studied before. In this reason, temperature of SPM has beenexpected while H2SO4 and H2O2 solutions are supplied to the spray nozzle with various flow rate. Andbehaviors of fluid are expected while SPM is sprayed on the SiO2 deposited wafer. Also, for the verificationexperiments, experimental set-up and related results will be described.
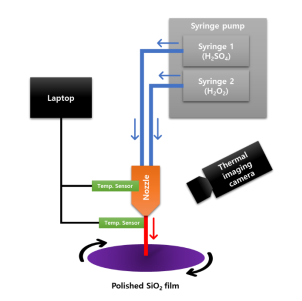
Fig1
The case1 – case 4 refer to flow ratio between sulfuric acid (99wt% H2SO4) and hydrogen peroxide (30wt%H2O2) as 3:1, 3.5:1, 4:1 and 5:1 respectively. The tendency in the reaction process is shown based on thesimulation results that the temperature increases fast and stays stable, which can be explained by thethermal effects of sulfuric acid dilution process, the main factor for the temperature change in this process,as well as the phase change triggered by the severe exothermic process. As the flow rate of the sulfuricacid increase, the settling time decreases, while the average temperature at outlet increases.
For the cleaning process, the thickness uniformity of SPM solution has correlation with the rotation speedwhich can be explained by the introduction of the stronger centrifugal force. The temperature distributionclosely depends on the flow rate and the temperature of the mixed solution. The temperature distributionon the wafer surface follows the same tendency as the thickness of the thin film of the solution, which canbe explained by the heat conduction between the wafer and the solution of high temperature. Apart fromthe mass of fluid transported, the influence of flow rate probably also comes from the intensity of theturbulence which will be studied in the future works.