Well-aligned ZnO nanorod arrays were synthesized by low-temperature wet chemical bath deposition (CBD)method on Si substrate under different conditions. Results illustrated that dense ZnO nanorods with hexagonalwurtzite structure were vertically well-aligned and uniformly distributed on the substrate. The effects of precursorconcentration and growth temperature on nanorods morphology were investigated systematically. The mechanismfor the effect of preparation parameters was elucidated based on the chemical process of CBD and basic nucleationtheory. It is demonstrated that the controllable growth of well-aligned ZnO nanorods can be realized by readilyadjusting the preparation parameters. Strong near-band edge ultraviolet (UV) emission were observed in roomtemperature absorbance spectra for the samples prepared under optimized parameters, yet the usually observeddefect related deep level emissions were nearly undetectable, indicating high optical quality ZnO nanorod arrayscould be achieved via this easy process chemical approach at low temperature.
CBD technique has been proven to be a good approach for synthesis of ZnO nanorods with the use of ZnO seeds inthe forms of thin films [16]. Here, Si was chosen as a substrate for ZnO nanorod arrays grown using a CBDtechnique. The Si substrates were cleaned in the ultrasonic bath with acetone, ethanol and deionized water to removeadsorbed dust and surface contamination. In order to fabricate vertically aligned nanorods, ZnO seed layer was firstfabricated on the substrates by simple silar method. 100ml aqueous solutions composed of zinc acetate(Zn(CH3COO)2.2H2O) with ammonia solution were used as a precursor source for the growth of ZnO nanorods. Allthe employed chemicals were analytical reagent grade. The concentration of Zn(CH3COO)2 .2H2O was varied in therange of 0.025 to 0.075M while keeping the molar ratio of Zn(CH3COO)2 .2H2O and ammonia to be 1:1. Thesolution was then transferred into a sealable glass beaker in which the substrates were suspended vertically. Theoptimized films with precursor concentration were taken for growth temperature variation in the range of 65°C to95°C. At the end of the growth, the substrates were taken out of the solution and rinsed several times with deionizedwater, and then blew dried with high purity Ar gas at room temperature. A detailed chemistry process by CBDtechnique can be found elsewhere.
A Philips Japan MPD 1880 X-ray powder diffractometer was employed to study the crystal structure of the films.Surface morphology of the films was examined by scanning electron microscopy, SEM (JEOL, 15 kV). A Shimadzudouble beam spectrophotometer was employed for obtaining absorbance in the wavelength range of 350–800 nmand to evaluate the direct band gap energies.
A series of experiments were performed by varying the precursor concentration butconstant to investigate the effect of the precursor concentration on thearrays. Fig. 2 shows the SEM images of ZnO nanorod arrays grown with the precursor0.025M to 0.075M. The growth temperature and timorphology of as-grown ZnO nanorod arrays are closely related to the precursor concentration.shows that initially for low precursoare small in size indicating that the growth anisotropy isanisotropy is constantly maintainedpossessed an average variation in nanorodorientation. The ZnO samples prepared from 0.025M and 0.05M zinc acetate solution possess hinanorod arrays with high density, which is due to the fact that the nucleation density on the Si substrate increases asthe Zn concentration in the solute on increases. As the solution concentration increases above 0.05M, it is veryobvious that the average diameter is dramatically increased by the clustering of nanorods, which leads to loweraspect ratio and may also worse the degree of nanorod orientation.concentration, are dense ZnO nanorod arrayuniformly distributed on the Si substrate.
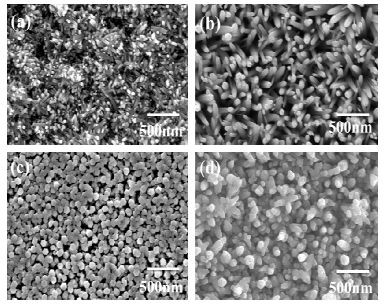
Fig1
The mechanism for the effect of precursor concentration and growth temperature can be elucidated with thechenical process of CBD and basic theory of crystal nucleation and growth. The chemical process involved in thegrowth of Zn0 nanorods can be described as follows: Zn(CH,COO)2.2H,0 provides Znions required for buildingup Zn0 nanorods, water molecules in the solution provide 0ions. Even though the exact function of ammoniasolution during the growth is stll unclear it is believed to act as a weak base. which would slowly hydrolvze in thewater solution and gradually produce OH' (20 . The Zn0 will form through dehydration of Zn(OH), and precipitateonto the substrates. leading to the formation of ZnO nanorods on the substrates.
Wurzite ZnO has polar surface such as (0 0 2), and nonpolar surfaces such as (1 0 ] and (1 0 0j. The aspect ratio ofthe as grown Zn0 nanorods will be determined by the relative growth rate of the polar surface and non-polarsurface. With the increase of precursor concentration, the amount of Zn(OH), produced from precursor solution willincrease correspondingly, which will increase the speed of growth during synthesis (2l]. These processes areendothermic and will hinder Zn0 nanorod arrays growth in the (0 0 2 directions, as a result, thicker nanorod arrayswere obtained under higher precursor concentration. The temperature dependent aspect ratio may also be understoodconsidering the fact that the relative growth rate of the polar (0 0 2) surface and the non-polar (l 0 1] and (! 0 0surfaces increase with the rise of growth temperature. Therefore, it is possible to control the morphology and aspectratio of ZnO nanorod arrays on the substrate by adjusting the precursor concentration, growth temperature and time.
下一篇: GaN薄膜的光学图案化