Abstract
Herein, we proposed a chemical mechanical polishing method for single-crystal SiC based on metal electrochemical corrosion and investigated the corrosion and wear performance of the Si face of single-crystal SiC. By comparing the corrosion performance of Al, Cu, and Fe metals on the Si face in a Na2SO4 electrolyte solution, it was found that only Al can generate a noticeable corrosion layer. The EDS and XPS analyses of the Si face confi rmed that the corrosion is due to the formation of the SiO2 layer. Frictional wear experiments were conducted to investigate the infl uence of solution composition on the wear behavior of Si face. Increasing the concentration of the Na2SO4 electrolyte solution resulted in higher wear, with a maximum wear value of 7.19 µm2 obtained in 1 mol/L Na2SO4 electrolyte solution. In an acidic corrosive solution, the Si face exhibited the highest material removal, with a wear value of 11.97 µm2 achieved at pH 3. The material removal mechanism of single-crystal SiC via metal electrochemical corrosion involved the corrosive reaction involving Al at the cathode, which generated a corrosion current, and the subsequent oxidation of the SiC surface at the anode, forming a SiO2 oxide layer leading to material removal.
Introduction
As a third-generation semiconductor material, single-crystal SiC with excellent physicochemical properties such as large forbidden bandwidth, high electron density and electron mobility, high critical breakdown voltage, high hardness, and chemical inertness towards strong acids and bases, is considered to be a revolutionary material in the fi eld of electronics and electricity in the future. High-power semiconductor devices made with singlecrystal SiC are widely used in satellite communications, aerospace, nuclear power generation, rail transportation, photovoltaic power generation, electric vehicles, and other electronic devices. The application of SiC-based materials for electronic device preparation and their epitaxial fi lm growth requires ultra-smooth surfaces free of damage and defects. However, their processing is challenging due to their physicochemical properties such as hardness, brittleness, and chemical stability.
Chemical mechanical polishing (CMP) is regarded as one of the most effi cient processing techniques for achieving global planarization of highly hard and brittle semiconductor materials. As a result of the chemical reaction between the polishing solution and the workpiece surface during the CMP technique, an oxide layer is formed on the workpiece surface. It is followed by the mechanical removal of the oxide layer through the combined action of a polishing pad and the abrasive. It is crucial to accelerate the chemical reaction rate on its surface to improve the material removal effi ciency of SiC. At present, commonly used strongly acidic or oxidizing solutions such as HF, H2SO4 , H2O2 , and KMnO4 exhibit low polishing effi ciency and the corrosive nature of the polishing solutions can be harmful to both the reactor and the environment. Therefore, the development of effi cient and environmentally friendly CMP methods, combined with the utilization of environmentally friendly polishing solutions, has received increased attention.
1 Experimental
1.1 Metal electrochemical corrosion
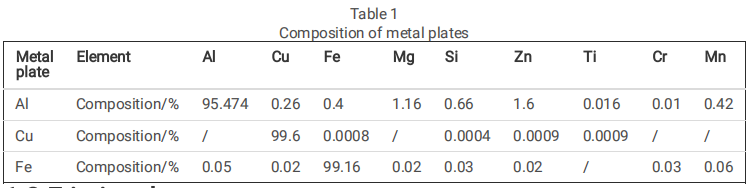
Static corrosion experiments with Al, Cu, and Fe metal plates(as shown in Table 1) with the Si face of singlecrystal SiC were conducted to determine whether the Si face can undergo electrochemical corrosion reactions with metals (see Figure.1). For use in the experiments, the metal plates were polished with sandpaper to remove the oxide layer, followed by ultrasonic cleaning. The plates were then dried under high-pressure air. The metal plates were then fi xed in contact with the Si face of single-crystal SiC using a non-conductive plastic fi xture. To compare the corrosion effect of different metals on the Si face, a portion of each of the metal plates was kept in contact with the Si face while being immersed in a solution of 1 wt.% Na2SO4 maintained at pH 7 for 1 h.
After the experiment involving the corrosion of the Si face, the Si face of single-crystal SiC was rinsed with deionized water and dried under low-pressure nitrogen gas before characterization. A scanning electron microscope (SEM, HITACHI-S3400N, Japan) was employed to observe the morphological changes of the Si face before and after the experiment. Elemental analysis of the Si face was conducted using an energydispersive spectrometer (EDS, Bruker-QUANTAX, Germany). Furthermore, the X-ray photoelectron spectroscope (XPS, Thermo Fisher-Escalab 250Xi, USA) was utilized to analyze the compounds formed on the Si face before and after the corrosion process.
1.2 Frictional wear test
As discussed in our previous work, the solution parameters in metal electrochemical corrosion have different effects on the C face of single-crystal SiC. Therefore, frictional wear experiments were conducted to study the wear performance of the Si face of single-crystal SiC under varying concentrations and pH of Na2SO4 electrolyte solution.
The experiments were performed using a frictional wear tester , as described in Table 2, and the apparatus setup is shown in Fig. 2. In the frictional wear experiment, a metal sphere made of Al (grinding ball) with a diameter of 5 mm was used (determined based on the experimental results in Section 2.1). The Si-face 4H-SiC single crystal (TanKeBlue Semiconductor Co. Ltd, Beijing, China) with an n-type dopant was selected, measuring 10 mm×10 mm in size, with an initial surface roughness of Ra 2 nm. Before the experiments, the SiC and the metal sphere were cleaned with alcohol and deionized water to remove surface impurities. The SiC wafer was then attached to the workpiece holder, and the metal sphere was fi xed on the frictional wear tester using a fi xture. A white light interferometer (BRUKER Contour GT-X, Germany) was used to examine the surface morphology of the SiC after wear. The cross-sectional area of the wear marks at eight uniformly distributed positions along the circumference of these marks on the SiC surface was calculated using Snipaste and Origin software. This cross-sectional area was used to evaluate the wear rate, the larger the cross-sectional area, the higher the wear rate.
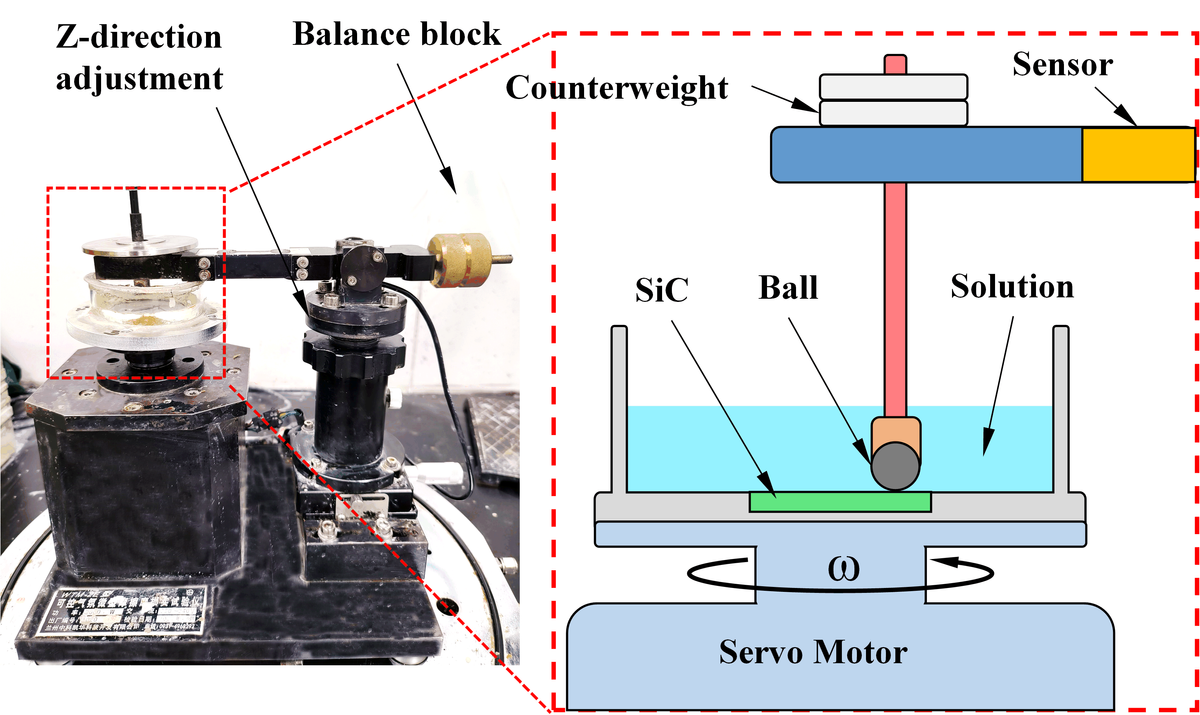
Figure 2 Setup for frictional wear experiments
2 Results and discussion
2.1 Infl uence of different metals on corrosion performance
The SEM and EDS results for the Si face before and after corrosion by different metal plates in the Na2SO4 solution are shown in Figure 3. Figure 3a presents the original surface morphology of SiC, exhibiting several scratches. EDS elemental analysis indicates the presence of only Si and C elements on the SiC surface (see Figure 3a2).
Figure 3b1 shows signifi cant corrosion cracking on the Si-face at contact with the Al metal plate, while no corrosion layer is seen in the non-contact region. On comparing the EDS elemental analysis spectra of the Si face before corrosion (Figure 3a2), the non-contact region (Point 1, representing the area of Si face not in contact with the metal plate), and the corroded layer region (Point 2, representing the area of Si face in contact with the metal plate), it is found that the non-contact region (Point 1) mainly consists of C and Si elements, with limited O and Al content. On the other hand, the corroded layer in the contact region (Point 2) exhibits a high O content (20.79%), a decrease in Si content to 76.62%, almost no detectable C, and the appearance of Al (2.49%). These results indicate that direct contact between the Si face and Al can result in metal electrochemical corrosion and the subsequent formation of the oxide layer.
Figure 3c shows the morphology of the Si face in contact with Cu. From Figure 3c1, dark spot-like uniform distribution can be observed on the Si face representing the Cu-Si contact region, suggesting a slight formation of the oxide layer. However, EDS elemental analysis reveals that the elemental content in the spotted region is nearly identical to that of the original Si face. The O content before and after corrosion is 0.77% and 0.35%, respectively, indicating no signifi cant oxidation on the SiC Si face. These results indicate that Cu exhibits weak metal electrochemical corrosion on the Si face in Na2SO4 solution, as there are no apparent indications of oxidation such as a signifi cant increase in O content.
Figure 3d presents the morphology of the Si face in contact with Fe. From Figure 3d1, it can be observed that the Si face remains relatively unaffected as compared to the original surface, with visible scratches present. EDS results show minimal variations in the C, O, Fe, and Si content of the Si face before and after the experiment and these slight variations can be attributed to the measurement error of the EDS instrument, suggesting that no signifi cant oxidation reaction occurred on the Si face during the experimental process. Similarly, these results indicate that Fe exhibits weak or no metal electrochemical corrosion on the Si face in Na2SO4 solution.
Figure 3 indicates that only the Si face of single-crystal SiC in contact with Al exhibits signifi cant electrochemical corrosion effects in the Na2SO4 solution. This is in stark contrast to the results observed for the C face of single-crystal SiC, where an oxide layer formed when the C face was kept in contact with Al, Cu, and Fe metal discs, with Al causing the most pronounced and thickest oxide layer. This highlights the distinct corrosion and processing characteristics of the Si and C faces of single-crystal SiC.
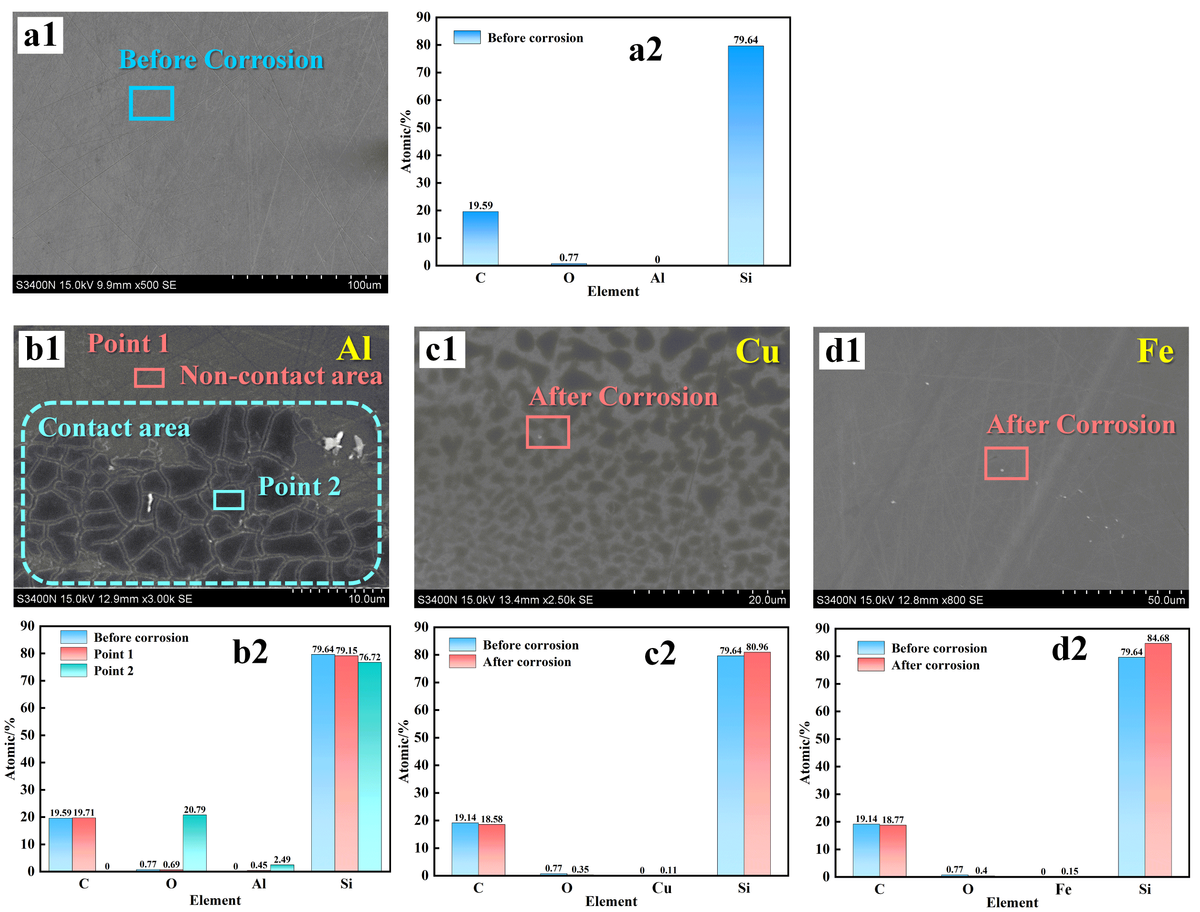
Figure 3 SEM images and EDS spectra of SiC before and after corrosion in the presence of different metal plates (a) before corrosion and SiC in contact with (b) Al, (c) Cu, and (d) Fe
3. Conclusions
In this study, a chemical mechanical polishing method for single-crystal SiC based on metal electrochemical corrosion was proposed, and the contact corrosion performance of different metals on Si face, and the wear performance of Si face under different solution parameters was investigated. The mechanism of material removal by metal electrochemical corrosion of single-crystal SiC was discussed. Based on the observation and results, the following conclusions were drawn:
(1) On comparing the corrosive effects of metals Al, Cu, and Fe on the Si face of single-crystal SiC, it was found that only Al had a signifi cant corrosive effect on the Si face, which caused the formation of the SiO2 layer on the Si face as confi rmed via EDS and XPS analyses.
(2) Na2SO4 electrolyte solution concentration and pH had a signifi cant infl uence on the wear performance of the Si face of SiC. The highest material removal capacity was obtained at a Na2SO4 concentration of 1 mol/L, with a wear cross-sectional area of 7.19 µm2 (137.3% higher than that in pure deionized water). Na2SO4 solution showed the highest material removal in strongly acidic environments, with a wear cross-sectional area of 11.97 µm2 at pH = 3 (37.3% higher than that at neutral pH = 7 and 106.4% higher than that at pH = 9).
(3) Galvanic coupling corrosion occurred as a result of contact between Al in Na2SO4 solution and SiC and the resulting corrosion current prompted the Si face anodic oxidation reaction to produce a SiO2 oxide layer. In a cyclic process, the fresh SiC surface is exposed to oxidation and is subjected to material removal, and effi cient material removal from the surface is achieved.