The purpose of the study is to determine the best technique for etching ZnO thin films. ZnO is deposited on the glass substrate using a radio frequency sputtering equipment. To etch the ZnO thin film, hydrogen peroxide (H2O2) concentrations of 10%, 20%, and 30% are utilised, with etching times of 30 and 60 seconds. The optical band gap is lowered after a specific quantity of etching, which shows that the film's crystallinity quality has improved. The impact of various ZnO thicknesses on the sample's optical properties is investigated using OPAL 2 simulator. In comparison to other ZnO layers of varied thickness, the OPAL 2 simulation shows that the 400 nm ZnO layer has the lowest transmission in the UV wavelength range.
1. Introduction
ZnO has developed many research interests for its applications in optoelectronic devices. Optoelectronics is the study and application of electronic devices and systems that detect and control light. In order for ZnO to be applied in optoelectronic devices, the fabrication of ZnO thin films on the numerous substrates plays a very important role. Examples of applications in the use of ZnO as a semiconductor type are UV light emitters, surface acoustic wave devices, gas sensing, piezoelectric interdigital transducers, self-powered UV photodetectors, supercapacitors, varistors, and solar cells. ZnO is also used in cosmetics as an ingredient in creams and lotions, as well as a UV filter in sunscreens. Besides, ZnO is used in a diversity of applications, commonly as an additive in products such as lubricants, glass, pharmaceuticals, batteries, paints, ferrites, cement, sealants, and plastics.
Substrates such as silicon, indium phosphide, calcium fluoride, and lithium tantalite were not chosen because of their high costs. Glass substrate is one of the most used because it is light in weight, has high transmission, and has high corrosion resistance. It can also be maintained at high temperatures. Soda lime glass substrate is one of the most frequent uses of glass substrate for fabrication and deposition of thin films. Soda lime glass consists of sand, which is 72.6%; soda is 13.9%; calcium oxide is 8.4%; magnesium oxide is 3.9%; alumina oxide is 1.1%; potassium oxide is 0.6%; sulphur trioxide is 0.2%; and iron oxide is 0.11%. The main attraction of this soda-lime glass substrate is that it is cheap and easy to get from ordinary stores. However, the disadvantages of this glass are that it cannot be used in strong acids and alkalis, and it is also easily cracked when the substrate is heated and cooled due to high thermal expansion. Soda lime glass substrate is used as a substrate because the fracture stress has a strong dependence on the time of loading and can be forced to load for a short period of time.
2. Experimental work
In this research, ZnO thin film is fabricated on a glass substrate by using the radio frequency (RF) sputtering technique. This method is chosen because it allows better control of deposition parameters such as substrate temperature and deposition rate. Before the substrate is fabricated with a ZnO layer, the glass substrate is ultrasonically cleaned in deionized water in order to avoid contamination on the substrate, which may cause low-quality thin film fabrication. The sputtered ZnO thin film on the glass substrates was etched with H2O2 solution at different concentrations (10%, 20%, and 30%). This method is chosen because it allows better control of deposition parameters such as substrate temperature and deposition rate. Before the substrate is fabricated with a ZnO layer, the glass substrate is ultrasonically cleaned in deionized water in order to avoid contamination on the substrate, which may cause low-quality thin film fabrication. The sputtered ZnO thin film on the glass substrates was etched with H2O2 solution at different concentrations (10%, 20%, and 30%) for the 30s and 60s etching times. To investigate the structural characteristics, surface morphology, refractive index, and thickness of the samples, UV-Visible Spectroscopy (UV-Vis), an optical microscope, a scanning electron microscope (SEM), and a filmetric are used. After that, the surface morphology of the samples is observed to determine the best etching rate with the selected concentrations. The OPAL 2 simulation from the PV lighthouse is used to verify the effect of ZnO thickness on the solar cell performance. Simulation OPAL 2 is used to study the ray tracing characteristics of ZnO on glass substrates under the solar spectrum AM1.5 G for the 300–1000 nm wavelength region. The samples' ability to transmit and absorb light is examined.
3. Result and discussion
Figure 1(a) is about the absorbance versus wavelength of 10%, 20%, and 30% concentrations of hydrogen peroxide (H2O2) is used as an etchant compared with glass, and asdeposited ZnO is referred to as glass and ZnO. The absorption of glass begins at 1.5 and decreases to 400 nm, as seen in the graph above. Meanwhile, absorbance of as deposited 10%, 20%, and 30% H2O2 concentrations follows a steady decline, then displays a steady increase from 310 nm to 360 nm, and finally shows a decrease towards 400 nm. Besides, the 30% H2O2 concentration resulted in higher absorbance compared to other samples. The higher absorbance value is due to the higher concentration of H2O2 which shows the absorbance works best with the concentration . The H2O2 acts as an oxidising agent and contributes to the surface roughness and formation of grain boundaries on the thin film. A higher surface roughness value will help the sample absorb light due to the formation of grain boundaries and a light trapping layer on the sample surface. Figure 1(b) is about transmittance versus wavelength for 10%, 20%, and 30% concentrations of hydrogen peroxide (H2O2). The transmittance of glass rises quickly toward 80% in Figure 1(b) and remains steady throughout the entire wavelength. Furthermore, the transmittance of the asdeposited, 10%, 20%, and 30% H2O2 concentrations exhibits a modest increase in pattern, followed by a fast increase, before levelling somewhere at the end of a spectrum. The glass has the highest transmittance due to its state of compressive stress .

Fig. 1. (a) absorbance and (b) transmittance of glass and ZnO thin films (30 sec)
Figure 2(a) is about absorbance versus wavelength for 10%, 20%, and 30% concentrations of hydrogen peroxide (H2O2). The absorbance of glass begins at 1.5 and decreases to 400 nm, as seen in the graph above. Meanwhile, absorbance of as deposited, 10%, 20%, and 30% H2O2 concentrations follows a downward trend, then displays a constant from 325 nm to 360 nm, and finally shows a decrease towards 400 nm. However, the 30% H2O2 sample produces the highest absorbance compared to other samples. The higher the concentration of H2O2 the higher the absorbance value of the sample. The 30% H2O2 sample produces a higher absorbance value when the wavelength increases. An oxidising agent, H2O2 is believed to produce rough surfaces and grain boundaries on the sample surface. Figure 2(b) indicates the transmittance versus wavelength for 10%, 20%, and 30% concentrations of hydrogen peroxide (H2O2). Glass transmittance is fast growing to 90% and becoming steady throughout the wavelength. Furthermore, all deposited samples exhibit a modest increase in trend before rising rapidly until they reach a stable condition at the wavelength's end. As the operating wavelength is extended, the transmittance value of the 30 percent H2O2 sample decreases.
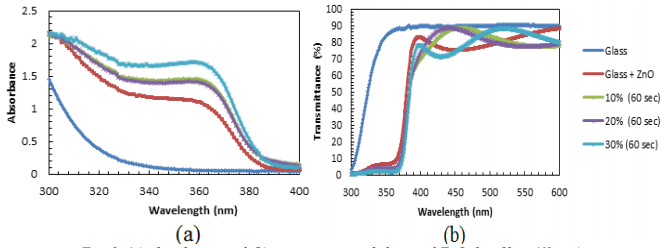
Fig. 2. (a) absorbance and (b) transmittance of glass and ZnO thin films (60 sec)
The 30% H2O2 sample has the highest absorbance and lowest transmittance compared to other samples. Figures 3(a)-(b) show the 30% H2O2 sample etched for 30s and 60s.The absorbance value of the 60s sample is higher than that of the other samples (see Figure 3(a)).Besides, Figure 3(b) indicates that the fabricated ZnO samples have a lower transmittance value compared with a glass substrate. Within the wavelength range of 300 to 430 nm, the 60s etched sample has a lower transmittance.

Fig. 3. (a) absorbance and (b) transmittance of glass and ZnO thin films (30% of H2O2)
4. Conclusion
The surface morphological and optical features of etched ZnO/glass have already been successfully examined at different H2O2 concentrations and etching times. Because light readily reflects and absorbs on the surface of the sample, a 30% H2O2 concentration for 60 seconds is optimal. After being etched for a certain amount of time, the optical band gap is reduced, indicating that the film's crystallinity quality has improved. The thickness and refractive index of the samples indicate that a higher concentration of H2O2 causes an increase in light absorption. The OPAL 2 simulation shows that, when compared to other ZnO layers of varied thickness, the 400 nm ZnO layer has the lowest transmission in the UV wavelength range.
上一篇: 碳化硅的新型光子应用
下一篇: 评估微流体流动条件下的微分颗粒变形能力