ABSTRACT:
Assessing the mechanical behavior of nano- and micron-scale particles with complex shapes is fundamental in drug delivery. Although different techniques are available to quantify the bulk stiffness in static conditions, there is still uncertainty in assessing particle deformability in dynamic conditions. Here, a microfluidic chip is designed, engineered, and validated as a platform to assess the mechanical behavior of fluid-borne particles. Specifically, potassium hydroxide (KOH) wet etching was used to realize a channel incorporating a series of micropillars (filtering modules) with different geometries and openings, acting as microfilters in the direction of the flow. These filtering modules were designed with progressively decreasing openings, ranging in size from about 5 down to 1 μm. Discoidal polymeric nanoconstructs (DPNs), with a diameter of 5.5 μm and a height of 400 nm, were realized with different poly(lactic-coglycolic acid) (PLGA) and poly(ethylene glycol) (PEG) ratios (PLGA/PEG), namely, 5:1 and 1:0, resulting in soft and rigid particles, respectively. Given the peculiar geometry of DPNs, the channel height was kept to 5 μm to limit particle tumbling or flipping along the flow. After thorough physicochemical and morphological characterization, DPNs were tested within the microfluidic chip to investigate their behavior under flow. As expected, most rigid DPNs were trapped in the first series of pillars, whereas soft DPNs were observed to cross multiple filtering modules and reach the micropillars with the smallest opening (1 μm). This experimental evidence was also supported by computational tools, where DPNs were modeled as a network of springs and beads immersed in a Newtonian fluid using the smoothed particle hydrodynamics (SPH) method. This preliminary study presents a combined experimental−computational framework to quantify, compare, and analyze the characteristics of particles having complex geometrical and mechanical attributes under flow conditions.
INTRODUCTION
In the field of drug delivery, various strategies have been employed to enhance the transport of nano/micron particles at biological target sites. Several independent parameters have been identified that would affect the transport efficiency, including chemical attributes (e.g., composition, surface charge, and targeting moieties); particle physical attributes (e.g., size, shape, and mechanical properties);and biological attributes at the target sites (e.g., expression of specific recognizing receptors, vascular and tissue architectures, cell phenotype, and genetic traits).From an engineering point of view, geometry and mechanical properties are key parameters as they can be modulated during the particle fabrication process. Moreover, while the geometry has been documented to directly impact the particle circulation half-life and tumor accumulation,the role of particle deformability is still under intense scrutiny. Furthermore, a variety of microscopy-based tools and flow-based techniques are currently available to accurately and rapidly characterize particle geometry, while no standardized test has been proposed to assess the mechanical properties of a micro/ nanoparticle under flow. Force spectroscopy techniques, such as atomic force microscopy,12 optical and magnetic tweezers,13 are far from being considered standards as they often provide operator- and machine-dependent results and, importantly, cannot provide quantitative data under flow.
Recently, microfluidics has been explored as a novel approach to address diverse issues ranging from performing on-chip biophysical experiments (lab-on-a-chip) to modeling complex biological processes, as well as a strategy to qualitatively examine the mechanical properties of nano- and micron objects.Indeed, microfluidics allows researchers to modulate, independently, a multitude of hydrodynamic parameters defining unique force, stress, and strain distribution regimens. For instance, the group of Di Carlo studied the role of the geometrical and mechanical properties using inertial microfluidics.Under relatively low Reynolds numbers, with Re ranging between 1 and 10, this technique defines the spatial position of particles as a function of their size and stiffness. In the same context, another approach was developed by Charrier et al. to determine the deformation mechanisms of healthy and sick red blood cells (RBCs) by forcing them to pass through orifices within a microfluidic channel.This study laid the basis for the quantification of the rigidity of nonaxial symmetric particles.
MATERIALS AND METHODS
Fabrication of the Microfluidic Filtering Device.
A microfluidic device was designed with a computer-aided design software (Layout-Editor) and realized using a soft lithographic approach. Silicon wafers with surface crystalline plane orientation <110> coated with a 500 nm layer of Ni3S4 (Si-Mat) were used. Figure S1a shows the orientation of the crystalline planes and the overall microfluidic chip dimensionalities. Figure S1b provides the steps required to realize the microfluidic filtering device during the fabrication process. Before lithography, a uniform layer of AZ 5214 positive resist (Microchem) was deposited over the silicon wafer via spin-coating at 4000 rpm for 1 min. The photoresist was cured on a hot plate at 110 °C for 1 min. The designed microfluidic pattern was transferred on the AZ 5214 positive resist using a Direct Laser Writer system (DLW6000, Heilderberg). Next, to develop the designed pattern, the silicon wafer was immersed in an AZ 726 MF developer (Microchem) for 1 min and then rinsed with water (step 1 of Figure S1b). Before transferring the pattern through the Ni3S4 layer, an oxygen plasma (O2) treatment (Plasma System Tucano, Gambetti) was performed for 120 s at 100 W and 0.5 mBar to clean the surface. The wafer was placed in an inductively coupled plasma-reactive ion etching (ICPRIE) (SI 500, SENTECH Instruments GmbH, cleanroom facility) to remove the 500 nm layer of Ni3S4 (step 2 of Figure S1b). Then, a rinse of acetone and isopropyl alcohol (IPA) was performed to remove the AZ 5214 resist, followed by water cleaning and an oxygen plasma treatment, as before (step 3 of Figure S1b). The wet-etching solution was prepared by mixing potassium hydroxide (KOH) (Merck) 30% (w:w), IPA 20% (w:w), and water 50% (w:w). The solution was then placed on a hot plate at 80 °C. The wafer was immersed in the solution for 12.5 min to obtain the final configuration and then rinsed with water (step 4 of Figure S1b). Note that the wetetching step took place once the solution achieved the set temperature. Then, to remove the Ni3S4 layer, a second wet-etching step was performed in Ceramic etchant A (Sigma-Aldrich) at 160 °C (Step 5 of Figure S1b). Finally, the surface of the silicon wafer was treated with perfluorotrichlorosilane (ThermoFisher) in a vacuum chamber for 1 h to facilitate the peeling off of the PDMS replica template.
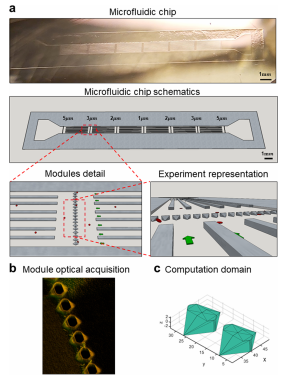
Figure 1. Microfluidic filtering device. a. Image (top) and 3D schematic reconstruction (bottom) of the microfluidic filtering device for assessing particle deformability under flow. The additional two representations at the bottom provide details on the filtering modules and showing the series of micropillars, spanning over the lateral width of the device, the supporting walls, and microparticles (red) transported through the filter openings by the flow. Green arrows indicate the flow x-direction. (b) Representative 3D confocal microscopy image depicting a series of micropillars with openings of 5 μm in the microfluidic filtering device. (c) CAD drawing of individual 5 μm opening, documenting the complex design obtained by the KOH wet-etching process in a silicon <110> wafer. This drawing defines the computational domain used for modeling.
RESULTS AND DISCUSSION
Geometry of the Microfluidic Filtering Device.
The microfluidic device, presented in Figure 1, comprises a single ∼2.0 cm long, 870 μm wide, and 5 μm high channel, with one inlet and one outlet, and 4 series of transversal micropillars constituting the filtering modules with openings of 5, 3, 2, and 1 μm. The microfluidic chip is symmetrically designed, as clearly shown in the image and 3D reconstruction of Figure 1a, so that the inlet and outlet can be readily reversed depending on the needs. The filtering modules are separated from each other in the x-direction by 2 mm to prevent any flow disturbance or fluctuation. Moreover, given the high aspect ratio of the channel, having a width/height (870:5) ratio of 160, supporting walls were interposed between sequential filtering modules to ensure structural stability of the soft PDMS replica of the microfluidic chip. Interposing these walls reduces the actual width/height ratio to 15. Importantly, as described in the Materials and Methods section, the lower base/curing agent ratio (4:1) with respect to the standard 10:1 strongly enhances the stiffness of the cured PDMS, thus leading to an enhanced structural stability.Given the imposed channel height of 5 μm (to prevent any tumbling motion of DPNs), the channel width was selected to be 870 μm to ensure a laminar flow and an effective movie acquisition.
Conventional 2D lithography allows one to realize accurate geometrical patterns at the micron scale but it becomes quite ineffective in the near 1 μm and submicron regime. The characteristic dimensions of the filtering modules and micropillars fall within this regime and, therefore, the anisotropic KOH wet etching of silicon was exploited to realize the device. Specifically, given the different etching rates along the crystalline planes, the KOH wet etching results in a typical 35.26° angle following the <111> (see Figure S1) crystalline planes for the x−y plane (flow plane). As to the z direction, there are two KOH wet etching processes. The first etching process shows an inclination of 35.26° parallel to the <110> crystalline planes, whereas the second etching process progresses perpendicularly to the x−y silicon plane and to the <110> crystalline planes, as seen in the fluorescence image of Figure 1b, technical drawings of Figures 1c and S2. Thus, the geometry of the microfluidic channel, the openings in the filtering modules, and the supporting walls can be accurately shaped at the micron and submicron scales, knowing the different etching rates between the crystalline planes. Clearly, the morphological variation showed after the KOH wetetching process (step 4 of Figure S1) needs to be predicted during the microfluidic drawing, as presented in steps 1−3 of Figure S1.
From the inlet (Figure 2a, left), inflowing particles encounter first the largest filtering module with an opening size of 5 μm, and then the second, third, and fourth filtering modules presenting openings of 3, 2, and 1 μm, respectively. In the silicon template, the micropillars appear as microwells and the supporting walls as longitudinally aligned microscopic slits (Figure 2a, right). Figure 2b shows scanning electron microscopy images of the arrays of microwells in the silicon template, and Figure 2c shows micropillars made of the PDMS replica. The opening size for the 4 sequential filtering modules is detailed in Figure 2c, reporting both the nominal and actual size as derived from multiple measurements (n = 17) conducted on the electron microscopy images. Specifically, a nominal opening of 5 μm corresponds to 4.9 ± 0.17 μm; 3 μm corresponds to 2.98 ± 0.076 μm; 2 μm corresponds to 2.03 ± 0.11 μm; and 1 μm corresponds to1.08 ± 0.1 μm.
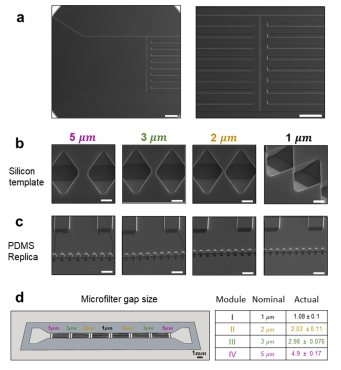
Figure 2. Geometrical characterization of the microfluidic filtering device. (a) Scanning electron microscopy (SEM) images of the silicon template, showing the inlet section (left) and magnified view of a series of microwells (right). Note that upstream and downstream of the microwells, a series of longitudinal microscopic slits equally spaced by 75 μm along the y-direction, are interposed (to avoid any structural collapse of the microfluidic channel). The silicon template is replicated into the actual microfluidic filtering device so that the microwells become micropillars and the longitudinal microscopic slits become the supporting walls. Scale bar: 200 μm. (b) SEM images of the microwells in the silicon template for the modules 5, 3, 2, and 1 μm. Scale bar: 5 μm. (c) Corresponding micropillars in the PDMS replica for all four filtering modules 5, 3, 2, and 1 μm. Scale bar: 25 μm. (d) Nominal and actual size of the openings in the four sequential filtering modules.
CONCLUSIONS
In this study, the mechanical properties of microparticles with a nonspherical shape were assessed under dynamic conditions using an ad-hoc designed microfluidic chip integrating multiple sequential filtering modules. A microfluidic filtering device presenting openings ranging from 5 to 1 μm was realized by finely controlling the KOH wet etching of silicon wafers, followed by conventional replica-molding techniques. The dynamics and mechanical behavior of 5.5 μm discoidal particles was experimentally determined within the microfluidic filtering devices and computationally modeled via a smooth particle hydrodynamic model. Experiments were conducted considering soft and rigid particles at four different controlled flow rates. sDPNs only were able to reach the smallest filtering modules, whereas the majority of the rDPNs were entrapped in the first module. Modeling allowed the authors to identify different particle behaviors in crossing the filter openings based on their initial location, deformability, and opening size, including the deformation of the particle without obstruction, temporary blocking, and permanent blocking. Interestingly, particles off-center from the filter openings experience hydrodynamic forces that would facilitate their rotation and crossing of the opening, even in the case of rigid particles nominally larger than the orifice. This study offers a computational−experimental framework for testing the mechanical behavior of microparticles under authentic flow conditions.
下一篇: 流速对微流控芯片中层流的影响