ABSTRACT
Charging rate of present lithium-ion batteries is needed to be fast for energy storage of electric vehicles. Lightassisted electrochemical reaction in lithium-ion batteries is one strategy to improve the rate capability. The original concept was reported by making use of the band bending between semiconductor electrodes and ionic conductive electrolytes under light irradiation. However, limited experimental data is provided by comparing light-assist lithiation using n-type and p-type semiconductors in lithium-ion battery system. Here, we report lightassisted electrochemical lithiation using p-type and n-type silicon single-crystals. Improved current density is observed in cyclic voltammetry under light irradiation. Silicon K-edge X-ray absorption spectroscopy confirms the enhanced lithium-silicon alloying reactions by light irradiation especially in p-type silicon. The electrochemical impedance spectroscopy shows that the charge-transfer resistance reduces under light irradiation, which indicates the kinetics acceleration of lithium ions at the electrode/electrolyte interface.
1. Introduction
Lithium-ion batteries which had been established in the last few decades, are well-known as one of the common types of energy storage systems . Recently, the widespread use of lithium-ion batteries in electric vehicles is expected to contribute to the reduction of greenhouse gas emissions. However, the present charging rate is approximately tens of minutes, which hinders the replacement of the conventional refuelling process. For the fundamental reaction in lithium-ion batteries, the electrode–electrolyte interface reaction in which the lithium-ion moves the interface has benn reported as the ratedetermining step. Nevertheless, a technique for effectively improving the charge and discharge rate of lithium-ion batteries has not yet been discovered.
Tributsch in the 1980s had proposed an interfacial photo-ionic reaction mechanism involving intercalation/deintercalation of ions which exhibits a potential for light energy conversion and storage using semiconductor materials. In this photo-intercalation concept, light provides the driving force to proceed with the interfacial ion transfer between electrode and electrolyte. Due to the potential difference between the electrode and electrolyte, the energy band bends to a higher energy level in p-type semiconductor and lower in n-type semiconductor. When the light irradiates a material with energy higher than the bandgap energy, the electrons from the valence band excite to the conduction band. In p-type semiconductors, this excitation concentrates electrons close to the interface, which accept the reaction with cations. When using lithium-ion electrolyte, lithium intercalation can be caused by light energy (Fig. 1 (a)). On the contrary, the photo-deintercalation process of cations can be caused in n-type semiconductors (Fig. 1(b)). The positive holes left on the valence band can move towards higher energy, then oxidize the atom to form cations that will be extracted from the semiconductor to the electrolyte.
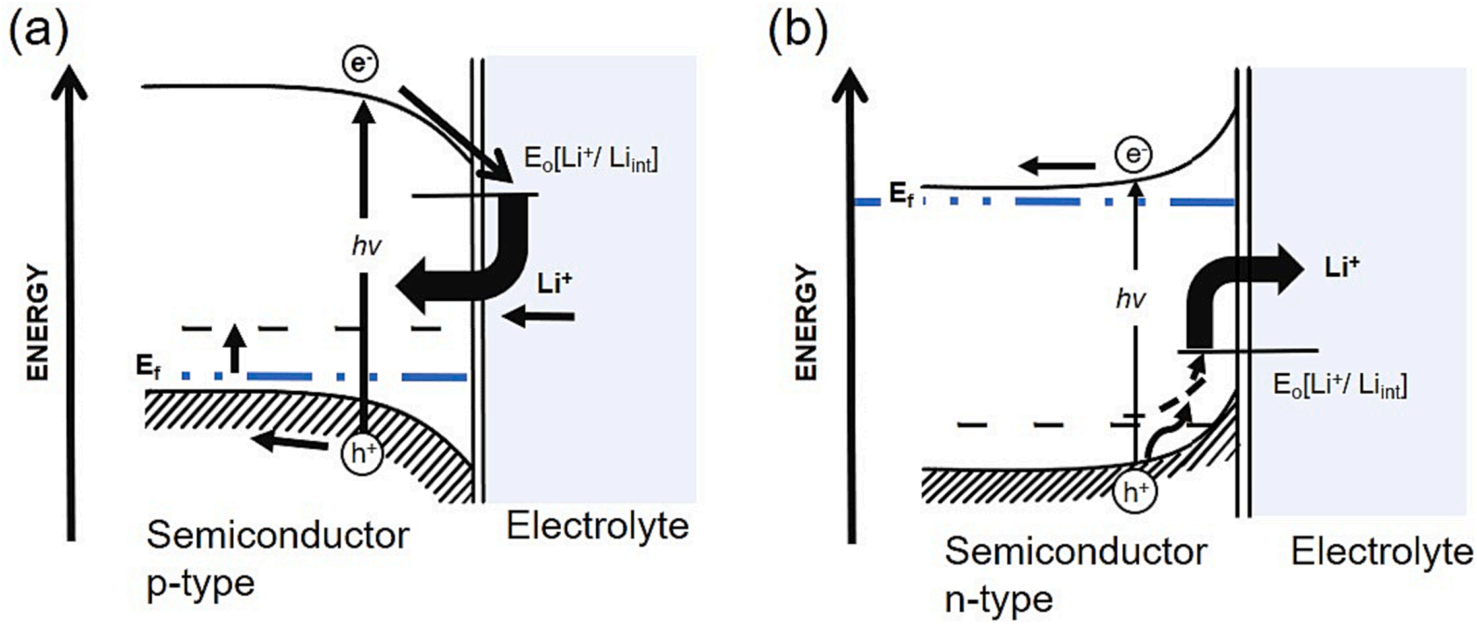
Fig. 1. Interface energetics for (a) p-type and (b) n-type semiconductors contacted with lithium-ion battery electrolytes based on the earlier proposed model by Tributsch
2. Materials and methods
2.1. Cell design and preparation
High-purity p-type and n-type Si (100) wafers were cut into rectangular to the size of 5x15 mm and underwent several pretreatment processes before assembling into the cell. The wafers were immersed in a hot piranha solution made up of a mixture of H2SO4 : H2O2 = 2:1 (v/v (%)) for 30 min to remove the organic contaminants at the surface of the semiconductor. The wafers were then dipped in 5 wt% of HF (46%) for 10 min to remove the surface oxide film. Each pretreatment process was always followed by a thorough rinsing using ultrapure water.
3. Results and discussion
To analyze the electrode potential during electrochemical measurement under light irradiation, a three-electrodes system in a transparent glass cell using liquid electrolyte and a Xenon lamp as the light source were used. The working electrode was placed facing to the light source so it could be irradiated by the light (Fig. 2(a)). The incident light that passes through the cell produces undesired heating, resulting in a change of cell temperature. Therefore, the cell was placed inside the temperature chamber to adequately measure the rise of the electrolyte temperature under light irradiation before conducting electrochemical measurements. Fig. 2(b) shows that the temperature continuously increases when the cell is irradiated without any initial temperature setting (black points in Fig. 2(b)). However, by controlling the ambient temperature from 20 ℃ to 50 ℃, gives a constant average increase of about 20 ℃ during prolonged light irradiation. Therefore, in subsequent electrochemical measurements, the measurement temperature at 20 ℃ under light irradiation and measurement at 40 ℃ in the absence of light were compared for each electrochemical measurement to satisfy the same temperature condition in the cell.
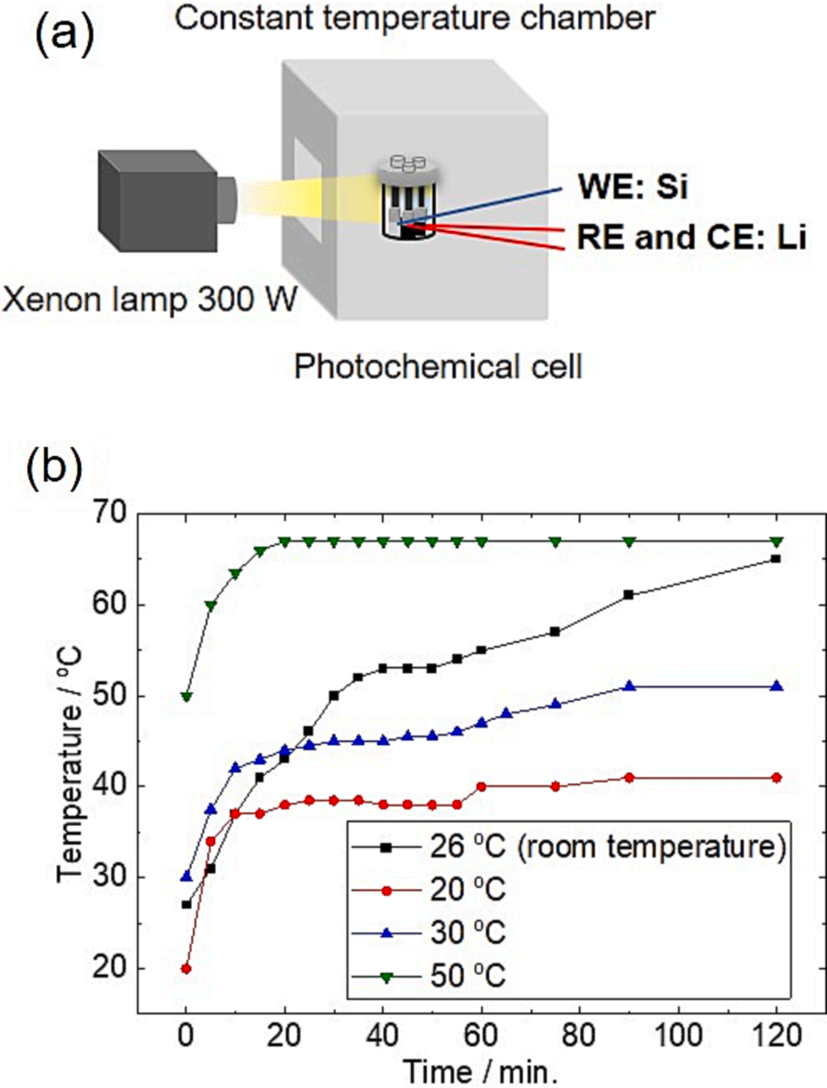
Fig. 2. (a) Schematic of the experimental set-up of photochemical measurements. The electrodes are facing directly to the light source and the cell is placed in the constant temperature chamber to control the ambient temperature. (b) Temperature changes of 1 M LiFSI electrolyte at various controlled initial temperatures by light irradiation. The plot at room temperature has no temperature control.
First, we discuss the effect of light irradiation during the potential sweep of p-type Si (p-Si) and n-type Si (n-Si) single-crystals. Fig. 3 (a) and (b) show CV profiles of p-Si and n-Si at 20 ℃ without light irradiation, at 20 ℃ under light irradiation and 40 ℃ at dark conditions, respectively. All CV measurements were performed independently, where the Si single-crystal electrodes without any past electrochemical treatment were used. The observed current reflects the electrochemical lithium alloying and dealloying reaction of Si electrodes. For the cathodic scan, the current density increases up to 0.01 V, which is set to prevent lithium deposition. The two anodic peaks at 0.4 V and 1.0 V correspond to the dealloying reaction of lithiated Si. We do not discuss the detailed peak assigned to the various alloying crystal structures in this study because the initial lithiation of crystalline Si forms an amorphous phase, which causes the complicated phase change during potential cycling. By comparing the cathodic peak profiles at three different conditions of both p-Si and n-Si, the light irradiation drastically improves cathodic and anodic current density. The observed coulomb charge during cathodic scan under light irradiation reaches up to 272 C for p-Si and 180 C for n-Si. Compared to the measurement at 40 ℃ at dark conditions that could only reach the coulomb charge of about 65 C for both p-Si and n-Si, the cathodic reaction under light irradiation is drastically improved. The photoexcited electron-hole provides more carrier species in Si semiconductors to proceed with the cathodic and anodic reactions. If the improved cathodic reaction is not originated from the lithium-alloying reaction but from surface film formation, the anodic current is not increased. However, the obvious improved anodic peak current indicates the enhanced lithiation into silicon anode by light irradiation. The later section will discuss this lithiated state based on Si K-edge X-ray absorption near edge structure.
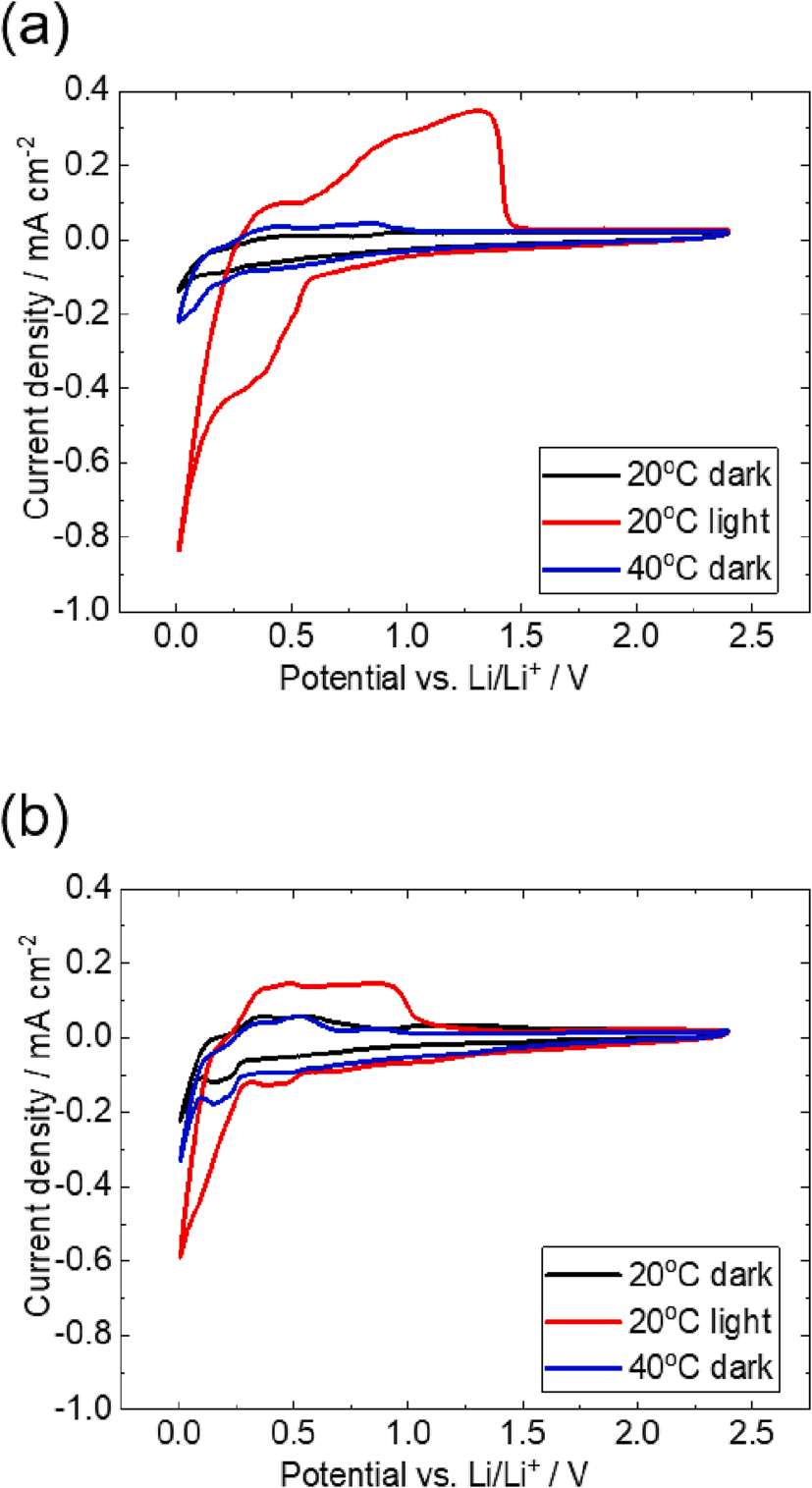
Fig. 3. Cyclic voltammetry (CV) curves of (a) p-Si and (b) n-Si single-crystals at scan rates 1 mV s − 1 over working potential range of 0.01 V to 2.40 V vs. Li/Li+ at 20 and 40 ℃ in the dark, and at 20 ℃ under light irradiation.
4. Conclusion
We have examined light-assisted electrochemical lithiation and delithiation that integrates Si single-crystal semiconductors as electrodes in a three-electrode system. The response of electrochemical lithium alloying and dealloying reactions under light irradiation is compared using p-Si and n-Si. Light irradiation significantly increases cathodic and anodic currents irrespective of p-Si and n-Si; reduction (lithiation) is more enhanced in p-Si than in n-Si. This phenomenon can be explained by the previously proposed band bending, in which electrons are concentrated near the interface in p-Si. Si K-edge XANES proves that the significant enhancement of the cathodic reaction in p-Si originated by lithium alloying reaction. Impedance measurements show that light irradiation reduces charge transfer resistance. These results indicate the possibility of reducing the rate-limiting interfacial reactions at electrode/electrolyte in lithium-ion batteries for a strategy of highrate charging.