We have evaluated some of the most recent breakthroughs in the synthesis and applications of graphene and graphene-based nanomaterials. This review includes three major categories. The first section consists of an overview of the structure and properties, including thermal, optical, and electrical transport. Recent developments in the synthesis techniques are elaborated in the second section. A number of top–down strategies for the synthesis of graphene, including exfoliation and chemical reduction of graphene oxide, are discussed. A few bottom–up synthesis methods for graphene are also covered, including thermal chemical vapor deposition, plasma-enhanced chemical vapor deposition, thermal decomposition of silicon, unzipping of carbon nanotubes, and others. The final section provides the recent innovations in graphene applications and the commercial availability of graphene-based devices.
INTRODUCTION
In 2004, Geim et al. synthesized a stable monolayer of graphene through mechanical stripping. Geim and Novoselov were awarded the Nobel Prize in Physics in 2010 for their successful experiments, and, subsequently, graphene became one of the world’s most studied two-dimensional materials. Graphene is a single atomic layer of carbon atoms arranged in a hexagonal pattern. It has a thickness of only 0.334 nm, making it the world’s thinnest material. It has numerous distinguishing qualities due to its unique properties, such as a large specific surface area (~2600 m2 /g),1 high electron mobility (200,000 cm2 /Vs),enhanced thermal conductivity (3000–5000 Wm/K),extreme optical transparency (97.4%),and exceptional mechanical strength, with a Young’s modulus of 1 TPa.
STRUCTURE AND PROPERTIES OF GRAPHENE
The number of layers of graphene regulates the different properties. SLG and BLG are zero band gap semiconductors owing to the encounter of the conduction and the valance bands at the Dirac points.A band gap can be opened in BLG by the application of an electric field. Furthermore, for FLG, the structure becomes more metallic with increasing layers. Hence, to comprehend the application of graphene, it is crucial to first understand the graphene properties. This section will review the structure and properties of graphene.
Structure of Graphene
SLG is a sp2 hybridized structure with two carbon atoms in a unit cell, and these two carbon atoms are located in the A and B positions, respectively (Fig. 1a). BLG can be divided primarily into three categories: AA-stacked, AB-stacked, and twisted(Fig. 1b). On the other hand, tri-layer graphene (TLG) has three stacking options. The AAA, ABA, and ABC stacking sequences represent the hexagonal, Bernal, and rhombohedral arrangements, respectively31 (Fig. 1c). Three out of four valence electrons for each carbon atom in graphene’s honeycomb lattice overlap with the adjacent carbon atom within the sheet, forming a strong sigma bond. The exceptional flexibility and robustness of graphene’s lattice structure are due to the sigma bond. The remaining fourth electron in the Pz orbital overlaps with the neighboring orbitals and forms a p bond that regulates the interaction between the graphene layers (Fig. 1d).
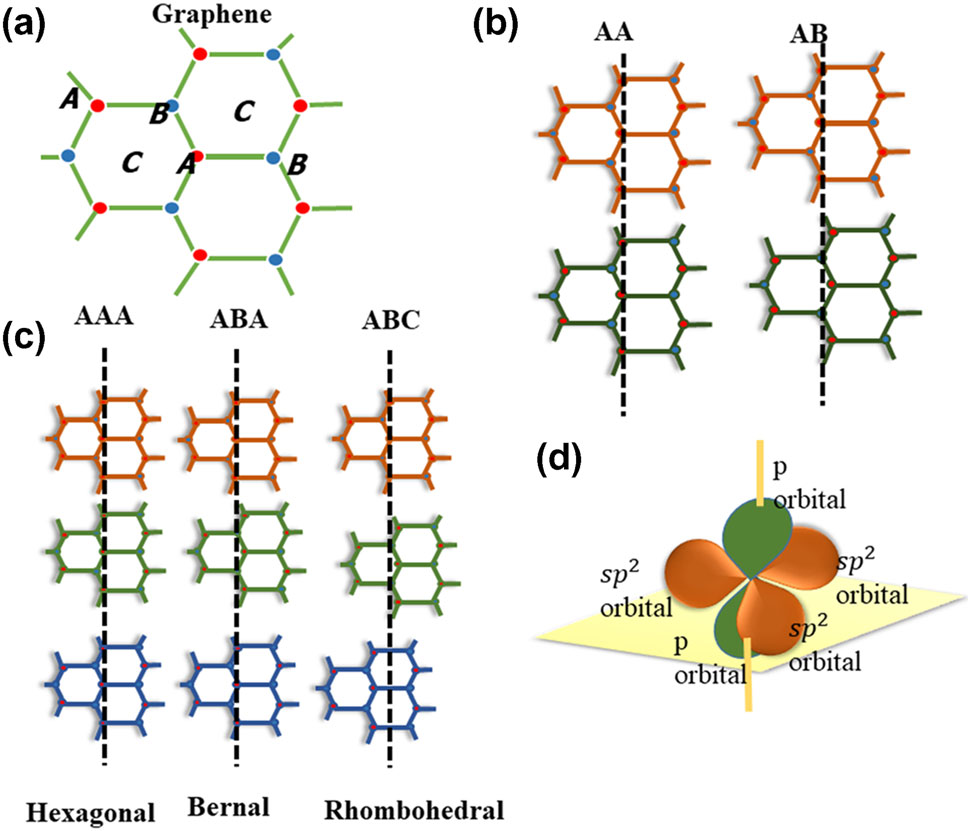
Fig. 1. (a) SLG structure, A and B denote carbon sites, (b) BLG stacking types, (c) TLG stacking types, (d) p bond and sigma bond positions in the graphene honeycomb lattice.
Properties of Graphene
Two linear bands in the band structure of the SLG cross at the Fermi level in the first Brillouin zone, which are Dirac points. Graphene is thus a zero-gap semiconductor (Fig. 2a).Holes and electrons near the Dirac point behave as massless fermions (m* = 0) and travel at extremely high speeds (106 m/s). SLG is recognized as a crucial material for electronics devices due to its outstanding properties and high flexibility. However, the zero-gap constrains its applications. Hence, the first obstacle to overcome is the opening of a gap. A perpendicular electric field applied across the graphene layer tunes the band gap of BLG and TLG due to different stacking sequences. Oostinga et al. demonstrated the change in carrier mobility from~1,000 cm2 /Vs to ~3,000 cm2 /Vs by tuning the gate voltage(Fig. 2b–c).
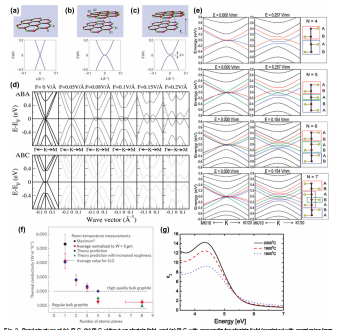
Fig. 2. Band structure of (a) SLG, (b) BLG without an electric field, and (c) BLG with perpendicular electric field (reprinted with permission from Ref. ), (d) ABA and ABC stacked TLGs under an electric field (reprinted with permission from Ref. ), (e) band structure of ABAB stacked FLGs under an electric field (reprinted with permission from Ref. ), (f) thermal conductivity of FLG observed as a function of the number of atomic planes (reprinted with permission from Ref. ), (g) imaginary part of graphene’s dielectric function as it has evolved at various growth temperatures (reprinted with permission from Ref. ).
FLG’s electronic structure is extremely sensitive to thickness, becoming increasingly metallic with increasing layers. Tang et al. investigated the effects of an electric field on FLG with layer numbers greater than 4. An external electric field can convert all ABC stacking graphene with N > 4 to semiconductors, whereas ABA stacking graphene remains semi-metallic (Fig. 2e).
RECENT ADVANCES IN GRAPHENE SYNTHESIS TECHNIQUES
Ruoff et al.separated the graphene layer from the graphite flakes using adhesive tape. Following exfoliation, these layers were dry-deposited on a silicon wafer, referred to as the ’Scotch tape’ method. Later, Novoselov and co-workers successfully synthesized a high-quality monolayer graphene by the Scotch tape method.Achieving high-quality graphene on a mass scale is one of the limiting issues to realizing its wide applications. The graphene synthesis techniques have been broadly classified in the following sections.
Exfoliation of Graphite
Even though the quality of graphene produced by Scotch tape or micromechanical cleavage is excellent,the yield is low, and the process is timeconsuming. As a result, this method is limited to laboratory research and appears impractical for industrial production. To improve the efficiency, Jayasena et al. reported a different mechanical cleavage approach for producing FLG from bulk graphite.The technique exfoliates graphite with an ultra-sharp single-crystal diamond wedge, followed by ultrasonic oscillations. Narayan et al. obtained about 30% yield of SLG and FLG sheets with only a few defects by directly sonicating graphite flakes in an aromatic semiconducting surfactant perylene tetracarboxylate (PTCA).They also observed that dried graphene/PTCA powders are easily re-dispersible in water.
Chemical Oxidation and Reduction Method
One of the traditional techniques for producing large amounts of graphene is the chemical reduction of GO. Initially, GO is produced via graphite oxidation using the modified Hummers method.Then, the prepared GO is reduced by hydrazine and sodium borohydride.The chemical reducing agents like hydrazine are not environmentally friendly and create hazardous by-products. Hence, researchers started to find safe and environmentally friendly reducing agents.
SUMMARY
A number of advantages of graphene, including its high surface area, chemical stability, thermal and electrical conductivities, super-hydrophobicity, flexibility, and so on have been discussed to illustrate the viability of bridging the nano-scale features of graphene to practical human-scale applications. To aid in the ongoing advancement of graphene-based materials and devices, this paper reviews the recent advances in their synthesis and applications. In terms of synthesis, various approaches have been successfully developed, indicating the feasibility of producing high-performance, high-quality, and large-area graphene. Exfoliation, chemical reduction of GO, CVD and thermal degradation of SiC, unzipping of CNTs, and other processes are among them. The CVD growth technique, among these, can generate large sheets of graphene with few or no defects and good conductivity; however, it must be taken into consideration that the quality of the material can be highly influenced by the process parameters used during the CVD growth. The exfoliation approach is compatible with industry-scale manufacturing of graphene powder, with certain methods yielding more than 50%. The qualities and performance of synthesized graphene have been demonstrated to be substantially reliant on the type of reducing agents and the synthetic process used. In terms of its practical uses, the properties of graphene have garnered much interest in a wide variety of different application domains. Graphene is commonly employed in transparent displays due to its excellent conductivity and flexibility. Because of its high sensitivity, graphene is utilized as a sensor in medical devices, particularly to detect certain viruses such as COVID-19 and malaria. Graphene is used in protective coatings for metals due to its corrosion and oxidation resistance, and, due to its high surface area and nonflammable nature, it is used as an electrode material for electrochemical energy storage devices, such as lithium batteries and supercapacitors to improve device performance when compared to those that use traditional carbon as electrode materials.