ABSTRACT
It is crucial to make Si wafer surfaces ultraclcan in order to realize low-temperature processing and high-selectivity in ultralarge scale integrated production. The ultraclean wafer surface must be perfectly free from particles, organic materials, metallic impurities, native oxides, surface microroughness, and adsorbed molecular impurities. Metallic contamination, the major type of contaminants to be overcome, has a fatal effect on device characteristics and must be suppressed to at least below 1010atom/cm ~. The current dry' processes, such as reactive ion etching or ion implantation, cause metallic contamination as high as 1010 atom/cm-. The wet process becomes increasingly important to remove these metallic impurities introduced during dry processing. Employing a new evaluation method, the metallic impurity segregation at the interface between the Si wafer and the liquid in the wet cleaning process was studied. It has been found that metals, such as Cu having higher electronegativity than Si, are directly adsorbed on the Si surface by taking an electron from the Si. On the other hand, metals, such as Fe and K having lower electroncgativity than Si, are not adsorbed on the Si surface. In the normal wet cleaning process when a native oxide is formed on the Si surface, metals such as Fe and K that are oxidized more easily than Si, are preferentially included into the native oxide. When the metals are in ultrapure water or chemicals with a concentration of 1 ppb, they are included into the Si surface, and native oxide with evaluation was 1012-1013 atom/cm2. Therefore, to decrease the metallic contamination level on the Si surface to levels less than 1x1010 atom/cm2, the metallic impurities must be suppressed to at least below the 1 ppt level in ultrapure water and high-purity HF, which are employed in the final step of the cleaning process. To prevent the metallic contamination on the wafer surface, it was found that it is important to maintain an inert atmosphere, such as N2 or Ar, to suppress native oxide growth and to reduce metallic impurities in the ultrapure water rinse. Moreover, it has been found that the diluted HF-H2O2 cleaning is effective in removing metals such as Cu, having high electronegativity, from the Si surface at room temperature and that it does not induce surface microroughness. This means the diluted HF cleaning, which has been employed in the final step of the conventional wet cleaning process to remove the native oxide, needs to be replaced with the diluted HF-H2O2 cleaning, tt was also found that surfactants added to improve the wettability of chemicals on the Si surface were also able to prevent metallic impurity precipitation on the wafer surface.
As the ultralargc scale integrated (ULSI) device featuring increasingly finer patterns is further integrated, it is becoming more critical to keep the Si wafer surface ultraclean in order to improve the device reliability and performance. The establishment of the advanced process technology requires the perfect control of the Si-gas interface and the Si-liquid interface. The introduction of total reflection x-ray fluorescence spectroscopy (TRXRF), scanning tunneling microscopy (STM), and atomic force microscopy (AFM) has made it possible to observe the metallic contamination and the microroughness on the wafer with high sensitivity.
Meanwhile in the current process, as shown in Fig. 1, the contamination on the Si wafer cannot be reduced to less than the level of 1012-1013 atom/cm =. These metallic contamination levels on the Si surface were measured with total reflection x-ray fluorescence spectrometry (TRXRF). At present, the removal of these metallic impurities introduced in the dry processing and the damages on the wafer surface entirely depend on the wet process. Therefore the wet process is required to be further improved.
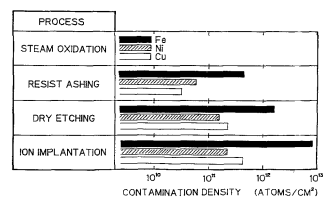
Fig. 1. Metallic impurities on silicon wafer surface from actual dry processing.
This article also discusses the efficiency of the DHFH202-H2O cleaning which has recently been proposed as a method of removing the metallic impuritiesJ . It has been found that the noble metals such as Cu, which cannot be removed solely by DHF, can be removed by DHF-H2O-H2O cleaning. The wettability of various chemicals onto the Si surface needs to be enhanced to clean thoroughly the wafer bearing very fine patterns. This is why the addition of surfactants is essential.This article also describes how the surfactant prevents metals in liquid from being adsorbed onto the Si surface: it is shown that when the surfactant is added to the chemical, the surfactant molecules are immediately adsorbed onto the Si surface, which prevents metals in liquid from being adsorbed onto the Si surface.
General Si Wafer Cleaning Method and Metallic Impurities
Generally the current Si wafer cleaning has five purposes: to remove organic materials in the H2SO4-H2O2-H2O cleaning, to remove particles and metallic impurities in the NH4OH-H2O2-H2O cleaning, to remove metallic impurities in the HCl-H2O2-H2O cleaning, to remove native oxide in the DHF-H2O2-H2O cleaning, and to remove the cleaning solutions in the ultrapure water rinsing. Table I shows the wet cleaning process of the Si wafer that we employ. Figure 2 shows the metal concentration on the Si wafer measured with TRXRF in each wet cleaning process step. It is clear that no metallic contamination is induced in each process step since the as-received wafers are sufficiently clear and the purity of chemicals and ultrapure water is sufficiently high.
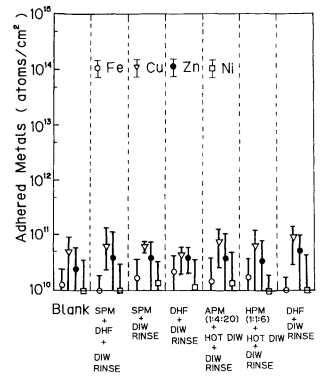
Fig. 2. Metallic impurities adhesion on silicon wafer surface during wet chemical cleaning.
Figure 3 shows the schematic diagram of the equipment for evaluating the metallic impurity segregations on the St-liquid interface. The vacuum chuck is installed in the chamber to support the wafer. The vacuum chuck is set so that the 6 in. wafer loaded on it is slightly bent with its center lower than the edge by 1 mm. The ambience in the chamber can be made inert by delivering high-purity nitrogen gas. It is also possible to make the ambience in the chamber atmosphere.
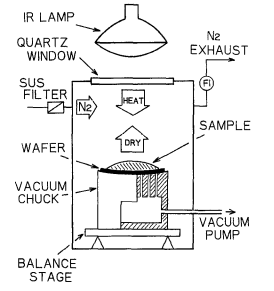
Fig. 3. Diagram of the equipment of metal segregation test.
Figure 4 shows the experimental procedure. The set amount of sample liquid is dropped onto the wafer surface and dried with an infrared lamp. The temperature of the droplet on the wafer rises to 50~ and stabilizes in 3-4 min. after the lamp is turned on. The sample liquid on the wafer is evaporated from the wafer edge first. The metallic impurities contained in the sample liquid, if they tend to adhere easily on the wafer, are segregated on the wafer surface from the liquid as the liquid is evaporated from the wafer edge. In this case, the metallic impurities are precipitated on the entire wafer including the wafer edge. The metallic impurities which have difficulty in adhering to the wafer remain in the liquid to get concentrated and carried to the center of the wafer by the liquid. After the sample liquid is dried up, the concentration distribution of metals adhering on the wafer surface is evaluated with TRXRF of 200 mA featuring the incident angle of 0.05 ~ and the x-ray excitation of 30 kV.
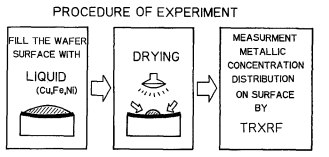
Fig4. Procedure of metal segregation test, liquid on the wafer isdried by lR lump healing. liquid temperature is 50°C.
Metallic impurities Segregation at the InterfaceBetween Ultrapure Water and Si Wafer
Figure 5 shows the drying process of ultrapure water on the Si surface in the clean air ambience. Figure 6 shows the drying process of ultrapure water on the Si surface in the nitrogen ambience9 Figures 7 and 8 show the change in diameter and weight of the liquid droplet over the time. Figure 9 presents the results of the same experiments performed using the Si surface covered with the native oxide.
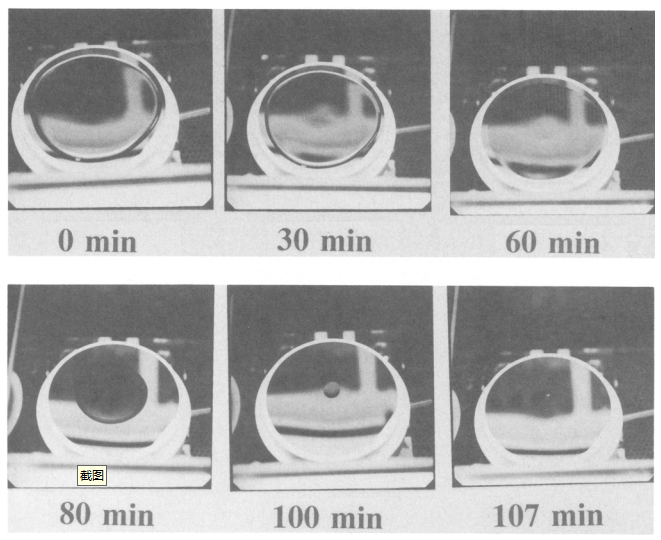
Fig. 5. Drying process of ulirapure water on the bare Si wafer surface in clean air (in air sealtype clean draft.
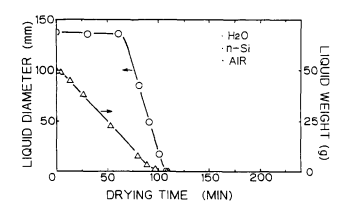
Fig.7. Diameter and weight of filled water on bare Si surface as afuncion of drying time in clean air.
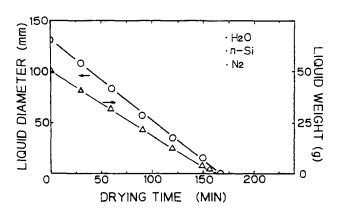
Fig. 8. Diameter and weight of filled water on bare Si surface as afunction of drying time in clean Nz.
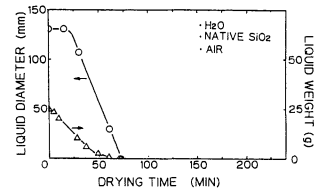
Fig. 9. Diameler and weight of filled water on native oxide surfaceas a function of drying time in clean air.
Conclusion
The metallic impurity levels at the Si surface higher than 1 x 1010 atom/cm2 have a fatal effect on the semiconductor device. In order to prevent the deterioration of the device characteristics, the metal contamination on the surface need to be suppressed to at least below 1 x 1010 atom/cm2. At present, the advanced wet cleaning process is indispensable for removing these metallic impurities on the wafer surface. It is a matter of course that metallic impurities must not adhere on the wafer surface at all from variouschemicals employed in the wet process.
The metallic segregations from the solution to the Si surface can be explained by the two mechanisms. The first mechanism is that metallic ions such as Cu featuring higher electronegativity than Si directly take the electron from the Si surface to be directly segregated on the bare Si surface. The second mechanism is that metals such as Fe which are more easily oxidized than Si are included into the native oxide growing on the Si surface to be segregated on the Si surface.
As the native oxide is formed on the surface during the ultrapure water rinsing, the APM cleaning and the HPM cleaning are conducted in the clean air ambience, the metallic impurities which are more easily oxidized than Si are segregated in the formed native oxide. The metals included in the native oxide which is to be segregated are easily removed in the diluted HF cleaning process. Ni, having slightly lower electronegativity than St, behaves in a different manner from Fe and Cu, but it can be eoneluded that Ni is mainly included in the native oxide and can be removed in the diluted HF cleaning.