Abstract
We report an efficient method for cleaning Ge substrates similar to the Ishizaka and Shiraki method for cleaning of Si. The Ge wafers are cleaned in running deionized water and etched with HF. A thin oxide layer was prepared by dipping in a mixture of H2O2 and H2O for a few seconds and the oxide layer was removed by dipping in HF. This procedure was repeated several times to ensure the removal of several atomic layers of Ge, Finally the thin oxide layer prepared as described above was thermally decomposed in ultrahigh vacuum by annealing in the temperature range 300-500°C. The resulting surface gave rise to sharp emission peaks due to surface states in UPS illustrating a clean surface. Impurities such as carbon and oxygen were below detection level in XPS and AES. Cross sectional TEM studies showed no defects associated with the cleaning procedure. Ge buffer layer growth and subsequent SiGe growth showed good morphology and no substrate/buffer defects.
Cleaning of semiconductor substrates is a very important aspect from the surface science as well as device fabrication point of view. Within the last decade or so Ge has emerged as a promising high performance device material owing to its narrower band gap, high hole mobility and high solubility limits for p-type dopants. There has been interest in growing heterostructures on Ge substrates as well. Surface cleaning employing Ar ion sputtering has been used by various groups. However, a major problem encountered by this procedure is the generation of defects though it can be minimized to a great extend by a moderate temperature anneal. Where as an easy and low temperature method exists for Si cleaning, cleaning of Ge substrate has been a problem in the literature. Recently Zhang et al. reported thermal desorption of ultraviolet-ozone oxidized Ge( 001) for the preparation of clean substrates. In this Letter we report an efficient and relatively easy method for the cleaning of Ge substrates.
The samples used in the present study are n-type wafers with a conductivity of l-5 R. cm. The UPS and XPS measurements are recorded using a VG CLAM 100 analyzer with He I and Mg Ka sources, respectively, in normal emission geometry. A VG UVL-HI with higher photon flux was employed for UPS measurements. Ex situ oxidized samples were loaded into the analysis chamber through a load lock chamber. The instruments are kept in a clean room. Typical time required to bring the samples from atmosphere to ultrahigh vacuum are 5-10 min. Typical pressure in the analysis chamber during XPS me~mements (15 kV, 20 mA) is of the order of 6 X lo- l1 Torr.
Firstly, the wafers are washed in running deionizedwater and rinsed in HF(HF:H2O in 9:1 ratio;although this ratio is not critical we feel a higher con-centration of HF is desirable) and then washed againin running water.It is to be noted that the wettingcharacteristics are different from that of Si wafers. Athin layer of oxide is prepared by dipping the samplein H202(H202:H20 in 9:1 ratio)for 10-15 s andthen washed in running water. The oxide layer is etchedoff by dipping the sample in the same solution of HFfor 5-10 s. This procedure is repeated 3-5 times. Theprocedure ensures the removal of several atomic layersof Ge. A final oxide layer is prepared the same way bydipping thc wafcr in H2O2 (H2O2: H2O in 9 : 1 ratio)for 10-15 s. The sample is dried by blowing N₂ gas andloaded in to the UHV chamber. All the wet cleaningprocesses are carried out in the same clean room wherethe analysis equipments are kept. The sample isannealed at 300'C for 20 30 min and annealed furtherat around 500°C for 1$ min, The two step annealing isfor the necessary out-gassing and subsequent oxidelayer decomposition.
In Fig. 1 we plot the He I UPS spectra of the oxidized Ge( 100) surface and that after the anneal. As seen, the oxidized surface exhibits a prominent peak at around 5.2 eV resulting from 0 2p states. The binding energy of this peak is different from that of the oxide on Si surfaces. This difference and related effects associated with the oxidation process of Ge surfaces will be discussed in detail in a forth coming paper. The surface, after annealing shows a prominent peak due to the Ge surface states due the presence of Ge dimers on the surface. This is similar to that of Si surfaces where a surface state peak is observed at about 0.82 eV below the valence band maximum. In the case of Si surfaces, the presence of Si dimers and the resultant surface state emission in UPS is considered a signature of clean surfaces. Following similar grounds, we attribute the existence of Ge dimer states to surface cleanliness. The possible impurities such as carbon and oxygen were below detection level in AES and XPS. The (2 X 1) LEED pattern observed after the annealing was of good quality. This demonstrates that the procedure described above is indeed an efficient and easy method for cleaning of Ge substrates. Furthermore, we observed clear dimer rows in STM images of Ge( 100) cleaned using this method.
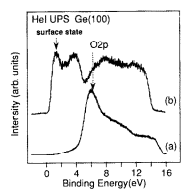
Fig. 1. He I UPS of (a) Ge( 100) oxidized by dipping the wafer in a mixture of HZ02 and H,O (9 : 1) at room temperature and (b) after annealing to 500°C showing the emergence of surface states. The wafer was cleaned prior to preparation of oxide as described in the text.
Cross sectional TEM measurements showed no defects associated with the cleaning procedure. Ge buffer layer growth and subsequent SiGe growth showed good morphology and no substrate/buffer defects. Although in cross sectional TEM we observe only relatively small interfacial areas (perhaps of the order of 100 pm2 in a typical sample) this observation establishes that substrate cleaning is effective. We have recently carried out misfit dislocation propagation kinetics in GexSi1-x/Ge( 100) heterostructures where the substrate cleaning was achieved using the method described here.
To conclude, we describe an efficient and easy method for cleaning of Ge substrates. Spectroscopic and microscopic evidences suggest that this method is indeed useful for subsequent growth studies.
上一篇: 下一代硅光子学路线图
下一篇: 通过界面钝化开发碳_硅异质结太阳能电池