ABSTRACT
Nanoporous materials have attracted extremely intense scientific attraction since the photoluminescence discovery at room temperature because of the quantum confinement effects. It is well known that in addition to the superior photoluminescence, nanoporous silicon materials prepared by the electrochemical method are promising materials for applications in catalysis, chemical, energy storage and biological sensing, due to its high porosity, modifiable surface, biocompatibility and biodegradability, which comprise with tunable optical porous Si structure and the applications such as biosensing, in vivo imaging, gas sensing and solar cells.
Therefore, the facile electrochemical methods employed to synthesize the nanoporous materials are marked, especially for nanoporous silicon materials aim to provide the crucial information about the relevant techniques to consider the eco-friendly developments for the future environmental risks to diminish.
Introduction
Recent years, the huge scientific research effort has been put into nanotechnology, and with the development of nanotechnology, the technology related to human is dramatically changed, even included an extensively profound influence on our daily life. It is well known that nanoporous materials are a subset of nanotechnology, which is also a signifcant class with captivating applications such as sensors, drug delivery, catalysis, electrodes and molecular separation. To date, the applications of nanoporous materials related to the biomedical have been extensively explored owing to its unique properties (such as tunable size of pores, large volume of pores, high specific surface area, feasible surface modification and chemical stability, etc.). Meanwhile, the structures of nanoporous materials also have fascinating conducting, magnetic and fluorescent properties resulted in attracting the abovementioned biomedical applications, for instance, optical sensors, electrochemical sensors, biomolecule determination, targeted therapy, drug encapsulation, controlled drug release, drug solubility improvement, theranostics, magnetic resonance imaging, fluorescent imaging, enzyme immobilization, gene transfer, nucleic acid protection, proteome analysis, adjuvants, implants, regeneration medicine, tissue engineering, etc.
Because of the contribution to the state-of-art synthesis strategies, bulk materials can be taken for preparing the porous materials with the cutting-edge techniques. Numerous types of pore morphologies along with the well-developed nanostructures have been proposed. Generally, pore morphologies consist of open pores which display a connection throughout the structure to the surface of the materials. Moreover, many different attractive pore shapes have been developed such as spherical, triangular, cylinder and sponge-like, etc. Furthermore, some nanostructured pores with the special characters, for instance, in the sinusoidal and wavy form can be explored with various controllable output waveforms fabricated by the electrochemical methods.
Methods for Synthesis of Nanoporous Materials Etching-Dealloying
Etching such as dealloying process refers to a chemical process in which the alloy is partially dissolved by the selective etching. In the alloys’ system, a less noble element is dissolved by the etchants and leaves behind a noble alloy constituent and an open nanoporous structure. The evolution of nanoporosity during the dealloying has been explored with the relevant results published in Nature. Study shows that the gold atoms are not dissolved and tend to cluster together to form Au islands, it opens up the pore and etches continuously throughout the bulk structure. Finally, the sponge-like porous Au is obtained after etching. Recently, fabricated a kind of nanoporous Au structures by dealloying Au/Ag. By HNO3 dealloying etching, the particles nanoporous keep the shape and the density of the surface density successfully along with the particles volume shrinking to some extent resulted in the lattice defects and the plastic deformation of Au crystal structure. It also indicates that the dealloying process is more efficient on the particles obtained by the liquid state process to obtain a more homogeneity of the AuAg alloy forming the particles.
Etching-Electrochemical Etching
In general, electrochemical etching is a common top-down approach to fabricate nanoporous materials. In electrolyte in two or three electrode configurations using a potenetiostat in this procedure, the bulk material is usually electrochemically etched, where by an applied voltage or current the pore is formed. The surface of the bulk materials reacts with the electrolyte (the etchants) to generate the pore structure and such reaction usually begins in the defect sites of the surface. Literature related to nanoporous materials by the electrochemical etching has been published: porous silicon (pSi), porous Ni, porous titania and porous alumina.
The texture beneficial to the cost-effective solar cells can be achieved easily by chemical and electrochemical etching (as shown in Figure 1) with the multidimensional and multilayers macroporous crater-like surface. Also, the correlated mathematical model of the macroporous silicon of the real layer was explored.

Figure 1: Different Geometrical Models with Macroporus Silicon Layers
Figure 2: FESEM images (a) top view (b) cross section of the porous silicon etched by ozone oxidization Porous silicon (pSi) has attracted the intense scientific research focus significantly since the discovery of photoluminescence at room temperature due to its quantum confinement effects. In addition to the photoluminescence of pSi, the applications (biosensing, in vivo imaging and gas sensing of other properties (high porosity, tailorable surface, biocompatibility and biodegradability) of pSi have been well exploited. Moreover, in the reflectance spectra the particular optical characteristics of pSi is extremely crucial to develop pSi-based sensor. It is well known that the single layer pSi displays Fabry-Pérot fringes and the modulated pSi multilayers with the waveform can fabricate into optical nanostructures for example Bragg stacks and rugated filters.
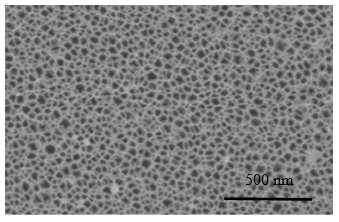
Figure 2a: Top view
Porous Silicon for Solar Energy Applications
It is all well-known that in the field of photovoltaics pSi materials have attracted much attention, especially for solar cells. The relevant advantages are listed as follows: (1) Ease and low-cost fabricating pSi. (2) Tuning the band gap from 1.47 to 1.8 eV by controlling the density of pores along with optimizing the sunlight absorption. (3) Enhancing light trapping and reduce reflection loss with increasing the short circuit current. (4) Converting solar radiation of shorter wavelengths into longer wavelength photons which absorbed more efficiently by bulk Si. More attractively all reported that the achieved efficiencies are over 20%, which one employed group IV reverse graded buffer layers grown on Ge/Si virtual substrates with a subsurface silicon porous layer to develop a GaAsP/SiGe tandem solar cell. And the latter took the silver assisted wet chemical etching to implement a simple and fast etching process yet effective for nano-scale texturing of mc-Si surface.
Conclusion
Due to the ease and quick fabrication by the electrochemical methods, the porous silicon (pSi) has the attractive optical properties with the controllable and tuneable porosity and pore size along with the enhanced morphological properties of the large internal surface area and the versatile surface chemistry. Owing to such unique properties of nanoporous materials (high porosity, modifiable surface, good biocompatibility and biodegradability), the nanoporous silicon materials prepared by the electrochemical methods will play more and more significant role in the field of catalysis, chemical, energy storage, gas sensing, biological sensing and in vivo imaging. Moreover, the above-mentioned captivating properties of pSi fabricated by the electrochemical methods definitely make the porous silicon a promising candidate for solar energy applications in the coming future. Meanwhile, for the future environmental risks and the sustainable development, the eco-friendly techniques shall be explored further because of the chemical usage.