SUMMARY
Micro-supercapacitors (MSCs) stand out in the field of micro energy storage devices due to their high power density, long cycle life, and environmental friendliness. The key to improving the electrochemical performance of MSCs is the selection of appropriate electrode materials. To date, both the composition and structure of electrode materials in MSCs have become a hot research topic, and it is urgent to compose a review to highlight the most important research achievements, major challenges, opportunities, and encouraging perspectives in this field. In this review, research background of MSCs is first reviewed followed by their working principles, structural classifications, and physiochemical and electrochemical characterization techniques. Next, various materials and preparation methods are summarized, and the relationship between the MSC performance and structure and composition of materials are discussed in depth. Finally, this review provides a comprehensive suggestion on accelerating the development of electrode materials to facilitate the commercialization of MSCs.
INTRODUCTION
With the emergence of portable technologies such as smart phones, implantable medical devices, and microsensors, their electrochemical energy storage components are similarly developing rapidly with a focus on miniaturization, integration, and flexibility toward use in field applications.Compared with traditional large-capacity power supply devices, micro energy storage devices are far more compatible with portable electronics due to small size and potential for high energy density. Therefore, the development of small energy storage devices matching with portable electronic products has become the development direction of the next generation of energy storage devices. To date, according to different charge storage characteristics, the available microscale energy storage units are divided into micro-batteries (MBs) and micro-supercapacitors (MSCs).Their total areal size can be in the millimeter or even the centimeter scale with a distance between two adjacent electrodes down to the micrometer scale. Contrary to MBs with low areal power density (<5 mW/cm2 ) and limited lifetime (<1000 cycles), MSCs can be fully charged or discharged in seconds, providing ultrahigh areal power density (>10 mW/cm2 ) and long cycle lifetime (>10000 cycles). Moreover, the diaphragm-free structure of MSCs can satisfy multidirectional high-efficiency ion diffusion while avoid the occurrence of short circuit. Additionally, they are safe to use, environmentally friendly, and easily compatible with wearable electronic fabrics and microelectronic integrated systems.
As a result, the field of MSCs has thus developed into an important class of miniaturized electrochemical energy storage devices In the past decade, the MSCs have been well studied and Figure 1 displays the development status of MSCs. In 2006, Sung et al. reported the polypyrrole (PPy) based flexible MSCs on solidified hydrogel substrate by photolithography and electrochemical polymerization.Since then, more and more research was studied on diversified electrode materials for MSCs.In the recent years, multi-functional MSCs with composite electrode materials have been explored intensively for practical applications. For example, just in 2023, Huijie Zhou et al. prepared the NiCo-MOF@CoOOH@V2O5 nanocomposites for MSCs fabricated by 3D-printed, exhibiting a high area specific capacitance of 585 mF/cm2 and energy density of 159.23 mW h/cm2 (at power density = 0.34 m W/cm2 ).
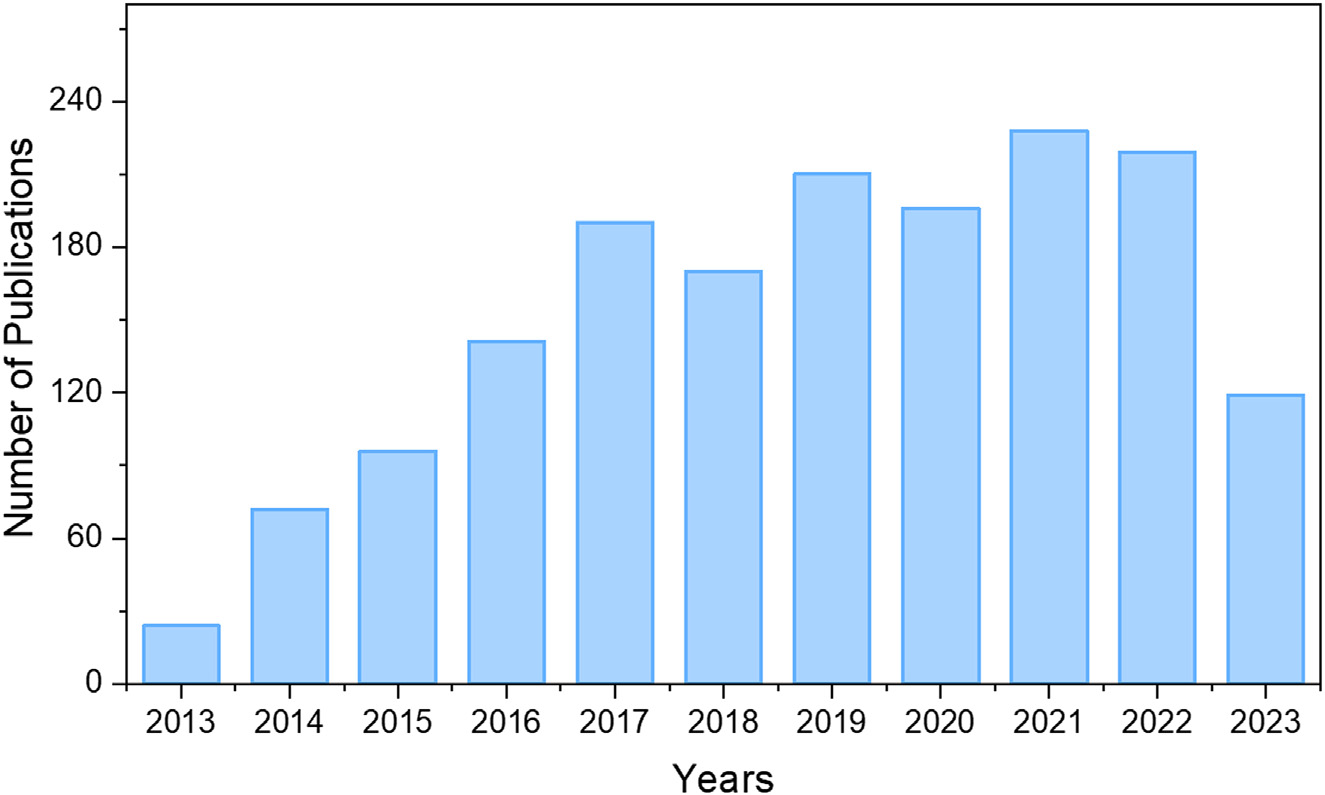
Figure 1. Number of publications on micro supercapacitors according to the Web of Science (as of July 31, 2023)
OVERVIEW OF MICRO-SUPERCAPACITORS
MSCs are a class of supercapacitors that feature a smaller device size but operate by the same working principle as supercapacitors. Also known as electrochemical capacitors, supercapacitors are a new type of energy storage device that possess qualities of both batteries and traditional plate capacitors. Energy storage is achieved either through electrical double layer capacitance (EDLC), which is generated by the adsorption and desorption process of charges on the electrode surface, or by the pseudo-capacitance generated by fast Faradaic reactions on the electrode material. The interfacial energy storage mechanism of supercapacitors requires a shorter time than battery materials for reversible redox reactions in the bulk phase, so supercapacitors possess higher power densities and excellent cycling stabilities.
Working principle of EDLC
The EDLC operates on the principle that upon the application of an electric field to the positive and negative electrodes, they will attract oppositely charged ions in the electrolyte to form a charge layer, thereby establishing an electric double layer and realizing charge storage.This principle is shown in Figure 3A. When the potentials applied to the two poles of the capacitor are different, the cations of the electrolyte will accumulate at the surface of the negatively polarized electrode material to balance the charge, while the anions will balance the positive electrode material. That is, the double layer exists at the interface between the electrode and the electrolyte ions. Through electrostatic adsorption, the positive and negative charges are separated, and charge layers of equal and opposite signs are generated to store energy. There is no charge transfer or redox reaction that occurs in this process, so the adsorption/desorption process at the interface can be rapid and the microstructure of the electrode material will not be damaged. This enables EDLCs to possess large power densities and long cycling lifetimes.
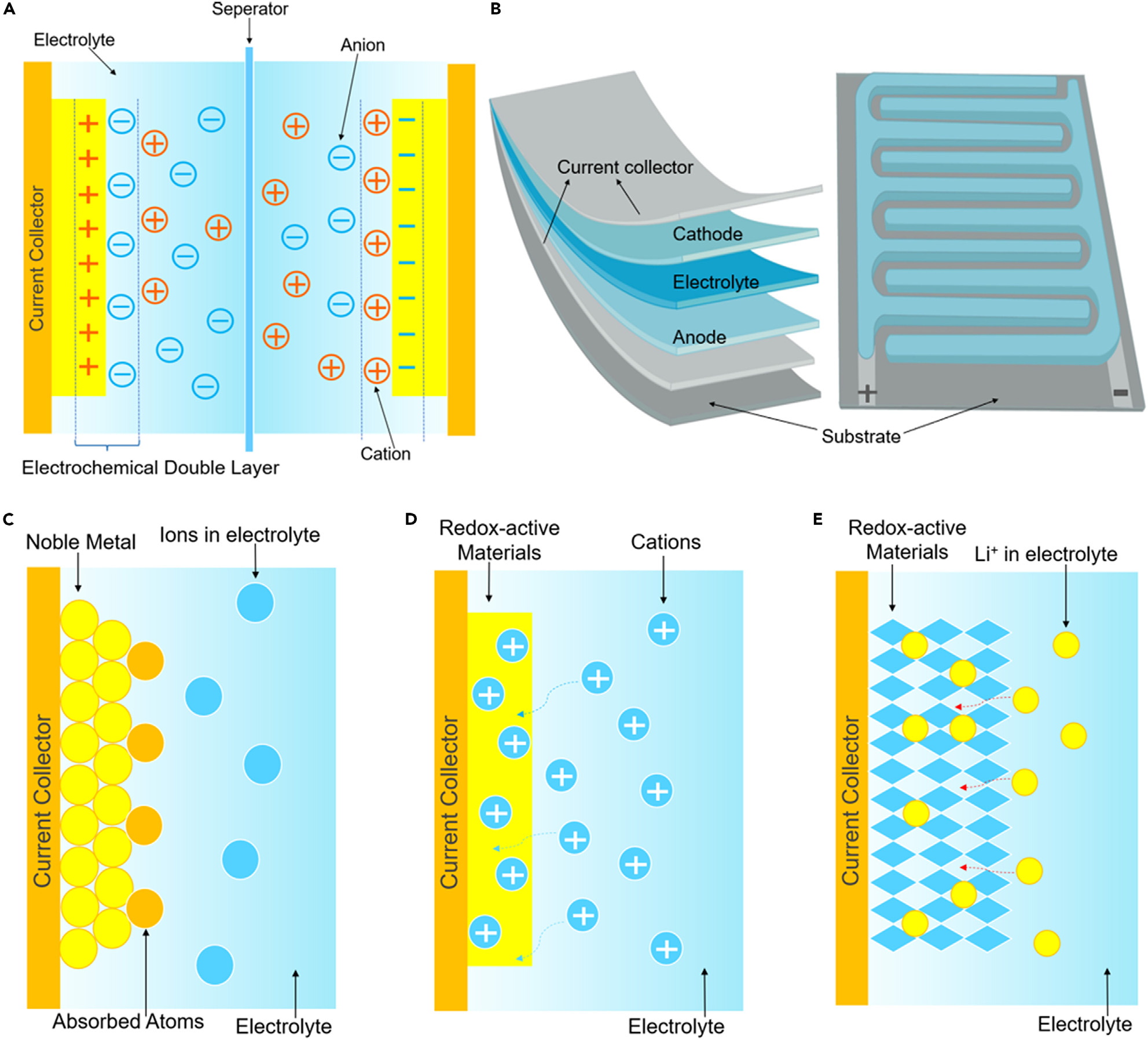
Figure 3. Principles and structures of electric double layer capacitors and pseudo-capacitors
PREPARATION PROCESSES
With the rapid development of MSCs, in order to adapt to different material characteristics and application fields, researchers have proposed various fabrication processes and methods.These can be mainly divided into two categories: The first describes a general method that requires separate preparation of metal current collectors in addition to active materials, and includes photolithography, electrochemical deposition, chemical vapor deposition (CVD), extrusion injection, etc. This kind of method can be applied to a wide range of materials, since the precision and resolution of the resulting interdigitated structure of the device are high and the devices are easier to miniaturize. However, because an additional current collector preparation process is required, the process flow is not ideally simple. Additionally, since the current collector and active material are prepared separately, the binding stability between the two is also an important issue.
下一篇: 有机电化学晶体管的加工