The surface treatment of glass-ceramic-based materials, namely, lithium disilicate glass (IPS e.max CAD), feldspar porcelain (VITABLOCS Mark II), and a polymer-infiltrated ceramic network (VITA ENAMIC), using aqueous fluoride solutions and their influence on luting agent bonding were investigated. Six experimental aqueous fluoride solutions were applied to these materials, and their effects were assessed by surface topological analysis. The obtained results were compared using non-parametric statistical analyses. Ammonium hydrogen fluoride (AHF) etchant demonstrated the greatest etching effect. Subsequent experiments focused on evaluating different concentrations of the AHF etchant for the bonding pretreatment of glass-ceramic-based materials with a luting agent (PANAVIA V5). AHF, particularly at concentrations above 5 wt%, effectively roughened the surfaces of the materials and improved the bonding performance. Notably, AHF at a concentration of 30 wt% exhibited a more pronounced effect on both etching and bonding capabilities compared to hydrofluoric acid.
INTRODUCTION
Recent advances in computer-aided design and manufacturing (CAD-CAM) systems have enhanced the production of dental prostheses. These systems can be used with various materials specifically developed for indirect tooth restoration1-3). Glass-ceramics, which are favored for their aesthetic appeal, robust mechanical and physicochemical properties, and biocompatibility, are prominent among these CAD-CAM materials4,5). Notable glass-ceramic types in the CAD-CAM domain include lithium disilicate glass and feldspar porcelain. In addition, polymer-infiltrated ceramic networks are categorized as glass-ceramic-based materials, as they are composed of a silicate glass-ceramic and resin6,7). Despite their advantageous features, glass-ceramicbased materials are brittle, which can lead to fractures in clinical settings8). To minimize such fractures, it is crucial to ensure a strong bond between the material and the abutment tooth. Effective bonding not only reduces the risk of fractures but also prevents material debonding9-11). Therefore, durable and reliable bonding is essential for the long-term success of tooth restorations using glass-ceramic-based materials.
Although HF is a highly effective surface etchant for glass-ceramic-based materials, its high toxicity and associated health risks are significant concerns. Consequently, there is an ongoing search for safer and less toxic chemical agents that could potentially replace HF. Among the alternatives explored, agents, such as phosphoric acid22-24) and fluoride-containing products (e.g., Monobond Etch&Prime®), have been investigated for their potential as surface conditioners. Despite their safer profiles, these alternatives have shown limited effectiveness in etching glass-ceramicbased surfaces; their etching abilities are inferior to those of HF. Because of these limitations, there is currently no alternative etchant that matches the efficacy of HF for the pretreatment of glass-ceramic-based material surfaces. This presents an ongoing challenge in the field, balancing the need for effective surface conditioning with the need for safer and less toxic materials.
MATERIALS AND METHODS
Experimental fluoride etchants
Table 1 presents the fluoride reagents selected for the preparation of the experimental fluoride etchants. Each etchant was prepared by dissolving a specific fluoride reagent in distilled water at 25°C, with continuous stirring using a magnetic stirrer. The resulting concentration of each solution was adjusted to approximately 80% of its saturation point in the aqueous solution. Table 2 presents the aqueous solutions derived from this process, which served as the experimental etchants: NH4F aqueous solution (AF), NaF aqueous solution (SF), SnF2 aqueous solution (TF), KHF2 aqueous solution (PHF), NaHF2 aqueous solution (SHF), and NH4HF2 aqueous solution (AHF). Specifically for the AHF etchant, diluted solutions were prepared at varying concentrations, namely, 1, 5, 10, 20, and 30 wt%, which were denoted as AHF1, AHF5, AHF10, AHF20, and AHF30, respectively.
CAD-CAM glass-ceramic based materials
Table 3 presents a list of the commercially available CADCAM blocks used in this study. Each block was sectioned into 2-mm-thick plates using a diamond wheel saw. The plates were then sequentially polished with emery paper of grit #1000 and subjected to ultrasonic cleaning in distilled water for 5 min. The polished and cleaned plates were used for the experimental procedures.
Etching procedure
The effectiveness of the fluoride etchants was assessed as follows. First, 20 μL of each experimental fluoride etchant was dropped onto the surface of the samples using a micropipette. The samples were left undisturbed for 60 or 120 s at 25°C. Subsequently, each sample was rinsed thoroughly with running water for 15 s, then ultrasonically cleaned in water for 5 min and finally dried using a blower for 30 s. The treated samples were characterized in subsequent experiments.
Shear bond strength (SBS) test
The SBS between the sample and luting agent was evaluated using the following procedure40). Each etched sample was secured in an acrylic tube using an autocured resin. A Teflon tube with an inner diameter of 5 mm was affixed to the etched surface of the sample using a doublesided tape to ensure a consistent bonding area of 19.6 mm². A commercial adhesive primer (Clearfil Ceramic Primer Plus, Kuraray Noritake Dental, Tokyo, Japan) was applied to the sample surface, according to the manufacturer’s instructions. A luting agent (PANAVIA V5, Kuraray Noritake Dental) was subsequently loaded onto the primer-treated surface to a height of 3 mm. This layer was then cured using a light irradiator (α LIGHT II N, J. Morita, Osaka, Japan) for 5 min, followed by a stabilization period of 1 h at 25°C. After removing the Teflon tube and tape, the bonded sample was immersed in distilled water at 37°C for 24 h. Thereafter, the samples underwent accelerated aging through 20,000 thermocycles, alternating between water baths at 5°C and 55°C for 60 s each, using a thermocycling machine (K178, TOKYO GIKEN, Tokyo, Japan). The samples with and without accelerated aging were subjected to SBS measurements using a mechanical testing machine (AGS-H, Shimadzu, Kyoto, Japan) at a crosshead speed of 1.0 mm/min (n=5). After the SBS tests, the debonded surfaces were examined using optical microscopy. The observed failure modes were categorized into two types: adhesive failure at the interface and cohesive failure within the sample.
RESULTS
Etching effects of various fluoride etchants The SEM images and corresponding surface roughness (Ra) values of the samples subjected to various fluoride etchant treatments are presented in Fig. 1 and Table 4. In the case of lithium disilicate glass, the surfaces experienced etching effects from the PHF, SHF, and AHF30 etchants. The Ra values for the samples treated with the SF, TF, PHF, SHF, and AHF30 etchants were significantly higher than those of the untreated samples. For feldspar porcelain, surface etching was observed with the AF, TF, SHF, and AHF30 etchants. The Ra values of the feldspar porcelain treated with AF, TF, and AHF30 etchants were significantly higher than those of the no-treated samples. For the polymer-infiltrated ceramic networks, the surface roughening was observed with the TF, SHF, and AHF30 etchants. The Ra values of the polymer-infiltrated ceramic networks treated with these etchants were significantly higher than that of the no-treated sample. These findings suggest that the AHF30 etchant is the most effective among the fluoride etchants examined for etching all the tested CAD-CAM glass-ceramic-based materials. Consequently, further detailed experiments were conducted using different concentrations of AHF etchants.
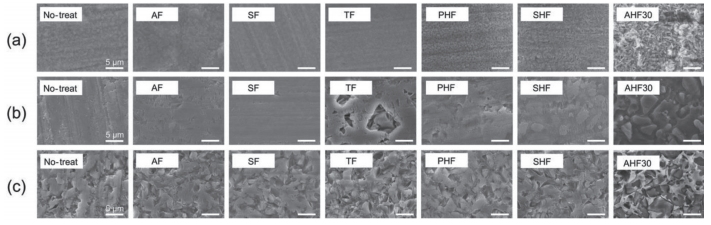
Fig. 1 SEM images for (a) lithium disilicate glasses, (b) feldspar porcelains, and (c) polymer-infiltrated ceramic networks after treatment with various etchants.
Influence of ammonium hydrogen fluoride etchant on bonding of lithium disilicate glass
Figure 2(a) shows the SEM images of the lithium disilicate glasses etched with varying concentrations of AHF etchants. The surface treatments resulted in increased surface roughness, and the degree of etching increased with increasing AHF concentration. Needlelike crystals were observed on the sample surfaces treated with AHF5, AHF10, AHF20, and AHF30 etchants. Particularly with AHF30 etchant, uneven surface etching was observed. The etched degree appeared to increase with the etching duration. This etching behavior was quantitatively assessed using the Ra values, as presented in Table 5. The Ra values of the lithium disilicate glass increased with increasing AHF concentration. Remarkably, AHF30 etchant demonstrated an Ra value of 1.891 μm, which is more than twice as large as that of the HF etchant (Ra=0.750 μm), thereby indicating that AHF30 etchant is the most effective concentration for etching lithium disilicate glass among the tested AHF concentrations. The SEM-EDX results presented in Fig. 3 show slight compositional variations on the AHF30-etched glass surface owing to its roughened topography; however, no evidence of segregation was detected. The SBS values of the etched lithium disilicate glass are listed in Table 6(a), and the corresponding failure modes are summarized in Table 6(b). No significant differences were observed between the groups that were not subjected to thermocycling (0 thermocycles). However, for groups that underwent 20,000 thermocycles, a notable difference was observed. The SBS values for the groups etched with AHF concentrations above 5 wt% were significantly higher than that of the no-treated group. Cohesive failure was uniquely observed in the AHF30 groups, both with and without thermocycling, suggesting robust bond formation between AHF30 and the luting agent.
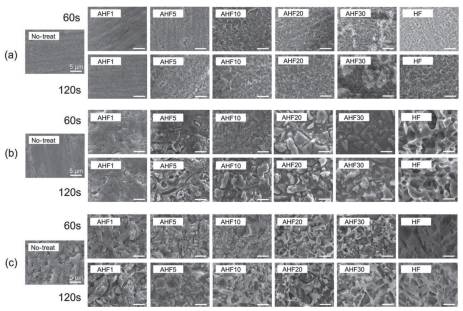
Fig. 2 SEM images for (a) lithium disilicate glasses, (b) feldspar porcelains, and (c) polymer-infiltrated ceramic networks, treated with AHF or HF etchants for the durations of 60 or 120 s.
DISCUSSION
The glass-ceramic-based materials employed in this study were lithium disilicate glass, feldspar porcelain, and polymer-infiltrated ceramic networks. Lithium disilicate glass, a high-strength glass-ceramic, incorporates needle-like lithium disilicate crystals within a silicate glass matrix4). Feldspar porcelain, a traditional glass-ceramic, is composed of alkali-aluminosilicate crystals embedded in a silicate glass matrix4). A polymer-infiltrated ceramic network, which is a hybrid of resin and glass-ceramic, consists of dual network structures comprising both alkali-aluminosilicate glass and acrylic resin components4). These glass-ceramicbased materials predominantly contain vitreous SiO2 as the main component. SiO2 can be etched with an HF aqueous solution according to the following chemical reaction41): SiO2+6HF→H2SiF6+2H2O. Consequently, the glass matrix of the materials dissolved in the aqueous HF solution render the surfaces of the materials rough.
In this study, we investigated the etching effects of six experimental fluoride etchants on glass-ceramic-based materials to ascertain whether etching similar to that with HF occurs. These fluorides were selected based on prior research on the etching of dental materials34-39). Our experimental results indicated that the AHF etchant exhibited the most effective etching of all the tested glass-ceramic-based materials among the examined experimental etchants. Chemical etching by the AHF etchant would be related to the fluoride species in the aqueous solution. Aqueous fluoride solutions, such as HF and AHF, contain fluoride species, such as F− , HF, and HF2 − , in their aqueous forms28,41-43). The etching mechanisms of SiO2 in hydrofluoric acid Among these species, HF and HF2 − can react with SiO2, effectively breaking the Si–O–Si bonds within vitreous SiO2 42). In contrast, the reactivity of F− is considered relatively low and negligible41). The proportion of these active fluoride species in an aqueous solution varies based on factors, such as the type of fluoride, its concentration, and the pH of the solution28,41,43). When comparing the range of fluoride etchants tested experimentally, AHF may have a larger ratio of such active chemical species than the other etchants. This elevated proportion of reactive fluoride species in AHF is believed to be responsible for the pronounced etching effect on the glass-ceramic based materials.
CONCLUSION
The aqueous solution of ammonium hydrogen fluoride effectively etched CAD/CAM glass-ceramic materials, namely lithium disilicate glass, feldspar porcelain and polymer-infiltrated ceramic networks. Shear bond strength between the materials and luting agent was significantly improved when using a solution with ammonium hydrogen fluoride concentrations above 5 wt%. A 30 wt% solution demonstrated a more pronounced impact on both etching and bonding compared to hydrofluoric acid.
上一篇: 促进二维材料的氟化和蚀刻