ABSTRACT
In this paper, atomic layer etching (ALE) processes for SiO2 are reviewed and categorized into two distinct group of anisotropic and isotropic ALE processes. Anisotropic ALE typically involves the fluorination of the silicon dioxide (SiO2 ) surface by fluorocarbon deposition during surface modification, followed by the removal of fluorinated layers by relatively low-energy ions. The impacts of the precursor, ion energy, selectivity, and chamber wall conditions on anisotropic ALE processes are reviewed. Isotropic ALE involves the conversion of SiO2 surfaces into a fluorinated layer or ammonium salt. This layer is subsequently eliminated through various chemical reactions, such as sublimation, fluorination, and ligand exchange. The mechanisms of etching in isotropic ALE are reviewed and classified into two subcategories of thermally isotropic and plasma-assisted isotropic ALE.
1. Introduction
Silicon dioxide (SiO2 ) has been applied as an insulator in various nanoscale semiconductor devices, playing a crucial role in the semiconductor industry for the past 50 years. SiO2 exhibits exceptional insulating performance, a bulk resistivity of 1015 Ω cm, and a dielectric breakdown strength of 107 V cm−1. Additionally, it is cost effective and easy to manufacture and demonstrates excellent compatibility with silicon bulk.
Plasma etching is a crucial and essential processing technique in semiconductor device fabrication. Its application to next-generation semiconductor devices is becoming increasingly challenging as the critical dimension (CD) of semiconductors decreases to 10 nm level. With decreasing CD and the adoption of three-dimensional structures, conventional reactive-ion etching processes are facing limitations in thickness controllability, etch selectivity, and surface roughness at the nanoscale. Consequently, atomic layer etching (ALE) processes are under active development, offering atomic-level precision in layer removal, minimized surface roughness, and exceptional uniformity.
ALE is a cyclic process that facilitates the atomic-level removal of various layers through a modification step involving radicals or molecules, followed by a removal step utilizing ions or chemical reactions as shown in Fig. 1. ALE processes can provide precise thickness control, excellent surface roughness, and high uniformity at both atomic and nanometer scales. A typical ALE process comprises four steps. Initially, the precursor chemisorbs onto the substrate surface through a chemical reaction, which may or may not be self-limiting. The second step involves purging to remove any physically adsorbed reactants. In the third step, modified surface layers are removed, forming volatile etch products via energetic ions or chemi.
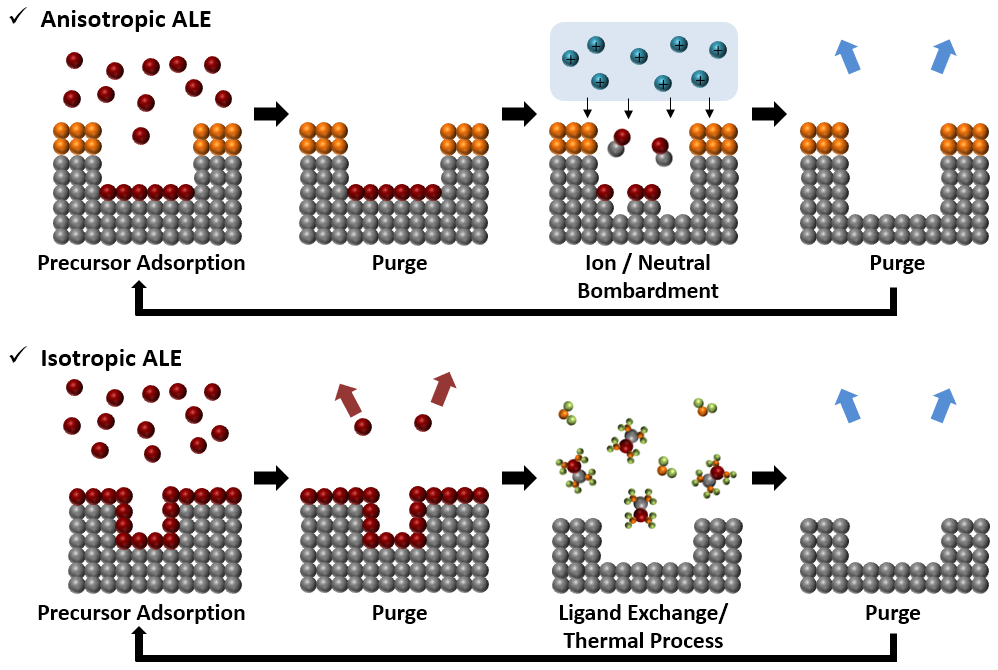
Figure 1. Comparative schematic of anisotropic and isotropic ALE processes for SiO2 .
The SiO2 ALE process utilizing C4H3F7O isomer plasmas was explored to elucidate the impact of chemical structure. The composition of the resulting fluorocarbon film is dictated by the chemical bonding structure of the C4H3F7O isomers, leading to variations in the F/C ratios as shown in Fig. 2. The F/C ratios of fluorocarbons generated by n-C3F7OCH3 and i-C3F7OCH3 plasmas are lower compared to those formed by CF3CF2CF2CH2OH plasma, which can be attributed to the CH3 radicals stemming from the presence of -OCH3 in the molecule. Correspondingly, the etching rate of SiO2 is highest in the CF3CF2CF2CH2OH plasma and lowest in the i-C3F7OCH3 plasma, mirroring the F/C ratio of the fluorocarbon films. It is observed that the addition of hydrogen to the plasma reduces the F/C ratio of fluorocarbon films and subsequently diminishes the etching rate of SiO2 .

Figure 2.
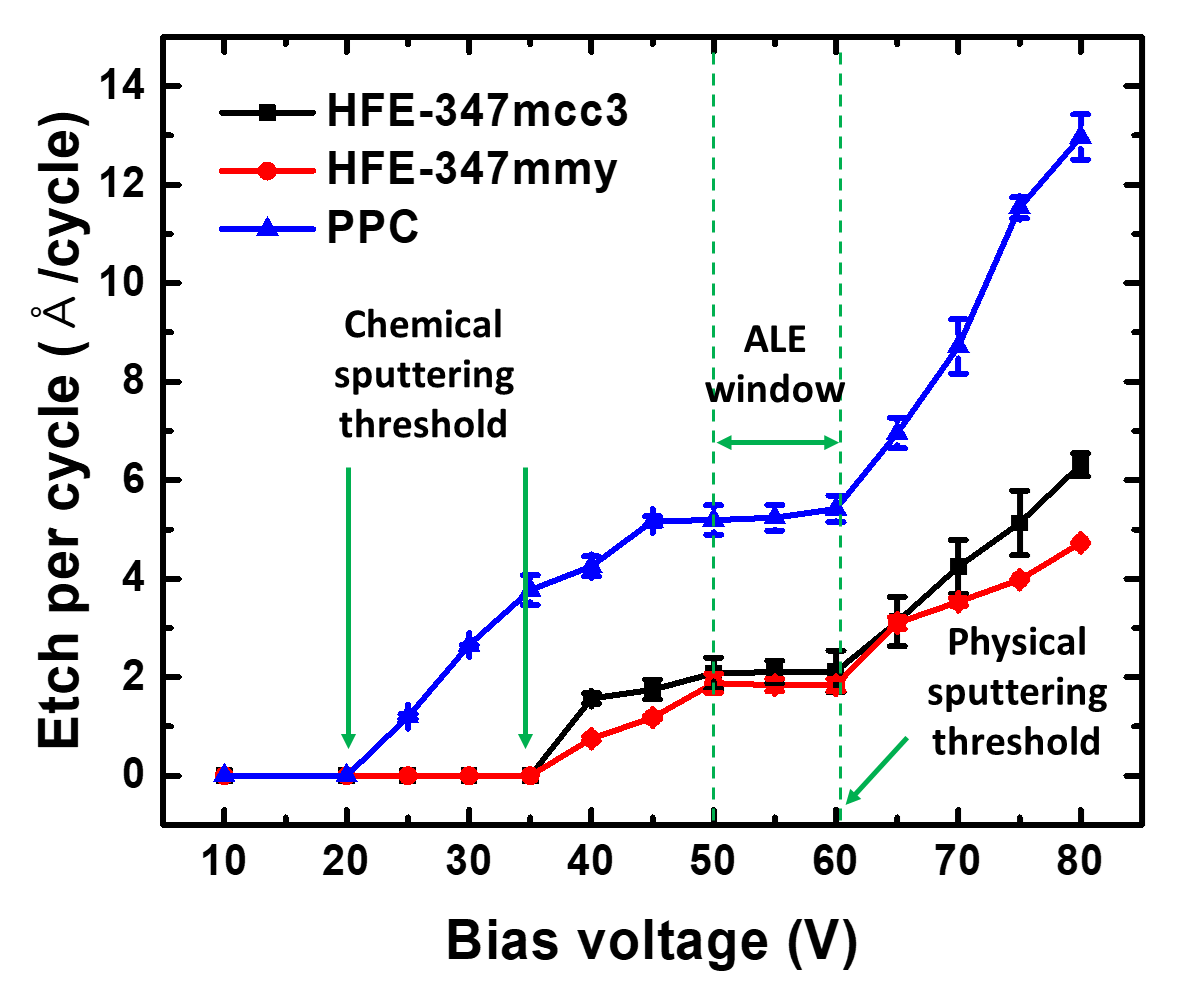
In the anisotropic SiO2 ALE process, the parameters defining the ALE window include the precursor used in the fluorination step, the type of gas employed in the removal step, and the ion energy, as summarized in Table II. The threshold physical sputtering energies identified are 50 eV for SiO2, 20 eV for Si3N4 , and 20 eV for Si. Investigations into the physical sputtering of SiO2 , based on the Ar bias voltage, revealed sputtering at ion energies above 65 eV. Surface modification allows for etching with lower ion energy and enables self-limiting removal of the modified layer. In the ALE window region, selective removal of the modified layer from the bulk material occurs. Three distinct regions are discerned based on ion energy: the incomplete etching region, the ALE window region, and the physical sputtering region, as shown in Fig. 3 . The removal of the modified layer is not complete at low ion energies, while sputtering of the underlying material occurs at higher ion energies.
The etching selectivity of SiO2 /Si and SiO2 /Si3N4 can be enhanced through various approaches, including the choice of precursors, control of fluorocarbon film thickness, ion energy management, etch step time adjustment, and selective deposition techniques, as outlined in Table III. The selectivity in anisotropic SiO2 ALE is influenced by the thickness of the fluorocarbon film, which in turn is determined by the choice of precursor. Improvements in SiO2 /Si and SiO2 /Si3N4 etch selectivity have been achieved using C4F8 , C4F8 /H2 , and C3H3F3 plasmas by studying the impact of hydrogen addition. The etching selectivity of SiO2 /Si and SiO2 /Si3N4 were notably improved using the C3H3F3 precursor, attributed to the reduction of fluorine concentration in the fluorocarbon film due to hydrogen. Additionally, the enhancement of SiO2 /Si etch selectivity has been discussed using C4H3F7O isomer plasma, as shown in Fig. 4. A higher presence of Si-C bonds was observed in the fluorocarbon films generated by the i-C3F7OCH3 plasma, compared to those from n-C3F7OCH3 and CF3CF2CF2CH2OH plasmas. These Si-C bonds act as inhibitors for Si etching, thereby decreasing the etching rate of Si and enhancing the SiO2 /Si etch selectivity. This evidence suggests that carefully selecting precursors can lead to high etch selectivity in anisotropic ALE processes. Moreover, selective functionalization of the SiNx surface with benzaldehyde has been shown to improve the etch selectivity of SiO2 /SiNx , as shown in Fig. 5. Benzaldehyde selectively deposits on SiNx surfaces featuring -NHx (x = 1, 2) groups, but not on SiO2 surfaces with -OH groups. This selective deposition of benzaldehyde on SiNx surfaces fosters the formation of a hydrofluorocarbon film, which serves as a barrier against the etching of SiNx . The implication of these findings is that through strategic precursor selection and surface functionalization, the etching selectivity for different material combinations in anisotropic ALE processes can be effectively manipulated and optimized.
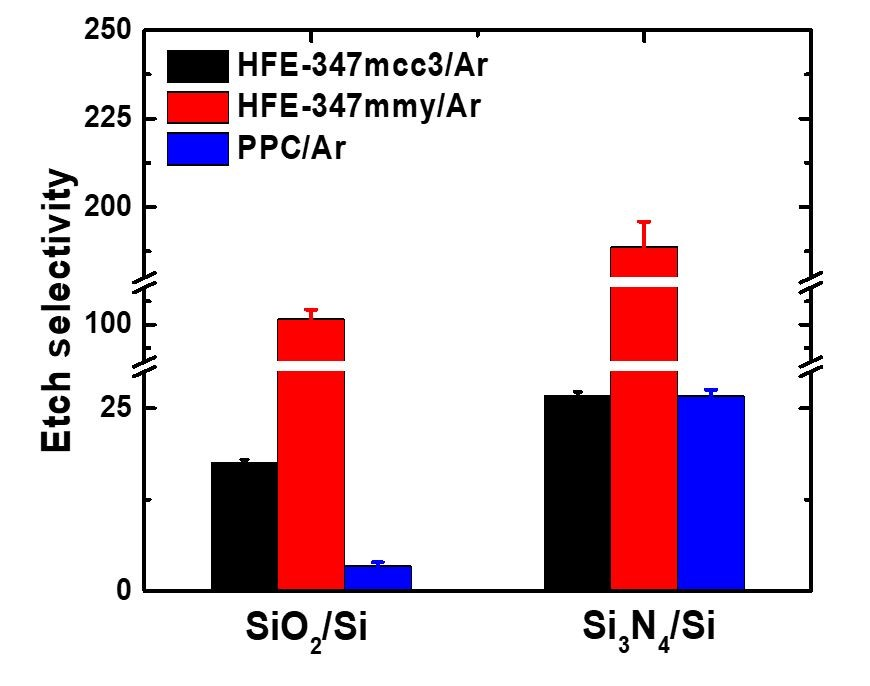
Figure 4. Precursor-dependent etching selectivity analysis for SiO2/Si and Si3N4/Si in fluorination step. Reproduced with permission from, Copyright 2023, American Chemical Society.
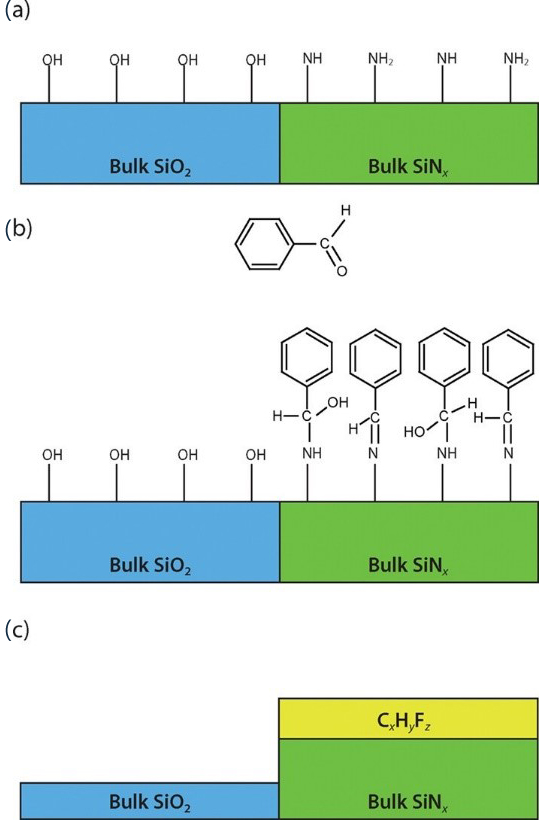
Figure 5. Selective ALE process of SiO2 over SiNx : (a) Initial functional groups on plasma-deposited surfaces; (b) selective functionalization of SiNx surface with benzaldehyde; (c) aromatic aldehyde aiding graphitic hydrofluorocarbon film formation on SiNx surface during ALE, inhibiting SiNx etching. Reproduced with permission from [38], Copyright 2021, AIP Publishing.
In the anisotropic SiO2 ALE process, the etching rate is influenced by the fluorocarbon film left on chamber walls, as summarized in Table IV. Achieving a consistent etching rate is crucial in ALE and the fluorocarbon film on the chamber walls plays a significant role in the repeatability of the etching rate. Variations in the SiO2 etching rate across multiple ALE cycles have been reported using in situ ellipsometry. It was observed that a fluorocarbon film deposited on the chamber wall leads to an increase in the etching rate as the cycle repetition increased. The impacts of the quartz window temperature and a fluorocarbon film on the chamber wall on the etching rate was reported as shown in Fig. 6. Research focusing on mitigating the chamber wall effect has been conducted using O2 plasma to maintain a constant etching rate. The application of O2 plasma effectively prevented the buildup of a fluorocarbon film on the chamber walls, thereby preserving the initial state of the chamber.
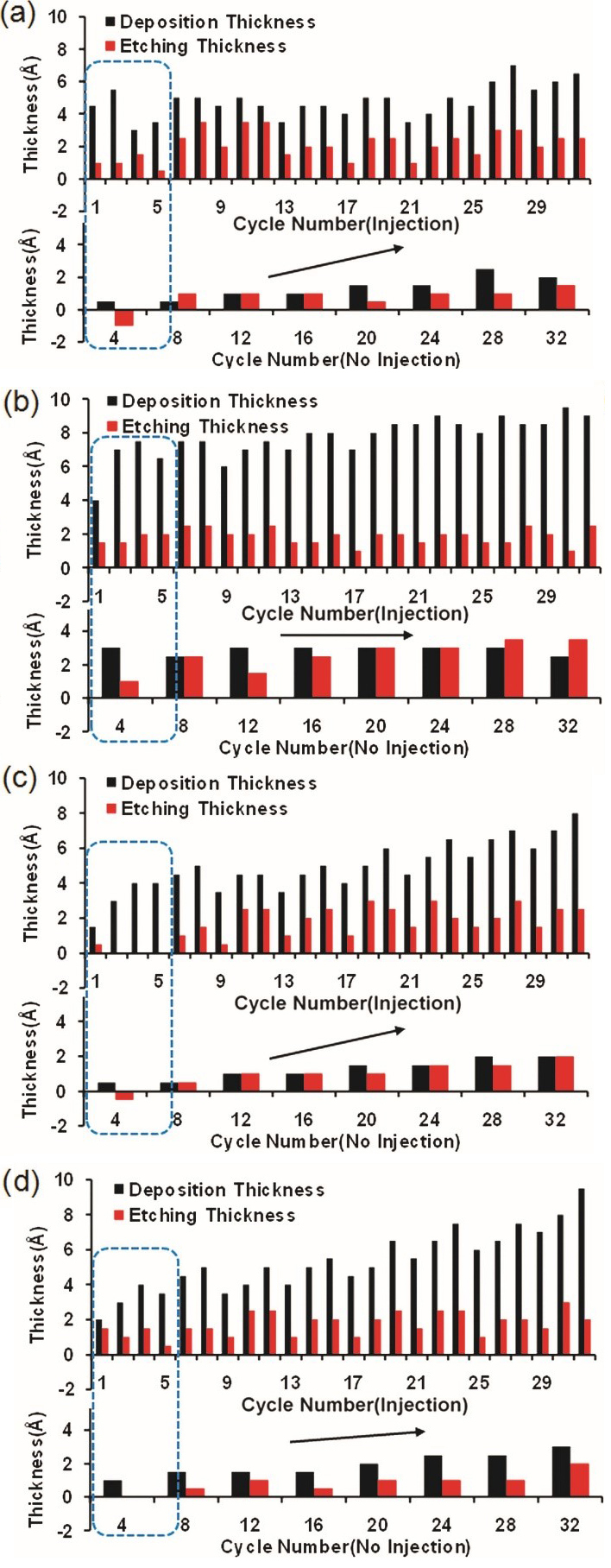
Figure 6. Analysis of deposition and etching thickness under four conditions: (a) Cold/clean, (b) cold/with film, (c) hot/clean, and (d) hot/with film. Reproduced with permission from, Copyright 2016, AIP Publishing.
The etching mechanisms of plasma-assisted isotropic SiO2 ALE are comprehensively summarized in Table VI. A specific isotropic SiO2 ALE process employing CF4 /NH3 or NF3 /NH3 plasma has been examined. In this process, SiO2 is converted to AFS in CF4 /NH3 or NF3 /NH3 plasma as shown in Eqs. (6) and (7). Subsequently, the AFS layer decomposes at temperatures above 160 ∘C, forming volatile substances such as NH3 , HF, and SiF4 , as shown in Eq. (8). The selflimiting property of AFS formation was confirmed to be dependent on the plasma duration, with the etching rate escalating from 2.7 to 7.0 nm/cycle based on the gas ratio, as shown in Fig. 7. The etching rate observed in the NF3 /NH3 plasma was approximately threefold higher than that in the CF4 /NH3 plasma. This discrepancy is attributed to the different bonding energies of F (~506 kJ/mol) and N-F (~239 kJ/mol). NF3 is more prone to dissociation than CF4 under similar conditions, leading to the generation of a greater number of fluorine atoms in the NF3 /NH3 plasma compared to the CF4 /NH3 plasma. This results in more extensive AFS formation and consequently, a higher etching rate. This step is crucial as it ensures the complete removal of the modified layer, enabling the process to proceed to the next cycle effectively. The distinction in etching rates between the two plasma types underscores the importance of gas selection and plasma conditions in optimizing the isotropic ALE process for SiO2 .
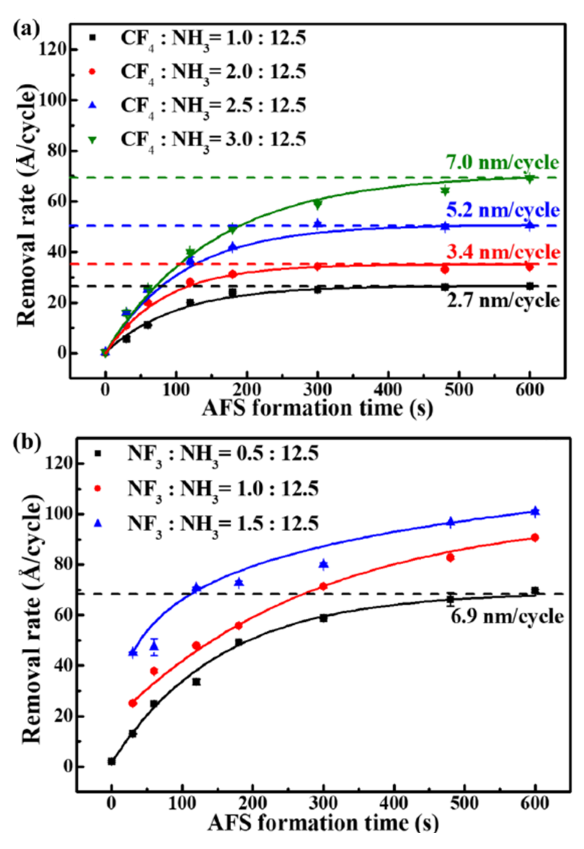
Figure 7. AFS formation time-dependent removal rates with (a) CF4/NH3 plasma and (b) NF3/NH3 plasma. Reproduced with permission from, Copyright 2020, AIP Publishing.
4. Conclusions
In this review, we categorized recent research on the SiO2 ALE process into anisotropic and isotropic processes. For anisotropic SiO2 ALE processes, the effects of the precursor, ion energy, selectivity, and chamber wall conditions were examined. The choice of precursor influenced the F/C ratio in the film deposited on the SiO2 surface, which subsequently affected the etching rate and selectivity. In the anisotropic ALE process, changes in ion energy affected the etching rate, and a consistent etching rate was observed within the defined ALE window. For isotropic SiO2 ALE, we elucidated two types of mechanisms are summarized. SiO2 surface into a fluorinated layer or ammonium salt, followed by removal through various chemical reactions, including sublimation, fluorination, and ligand exchange. Given the increasing complexity and three-dimensional nature of semiconductor device integration, the necessity for both anisotropic and isotropic ALE processes is evident. The selection of specific SiO2 ALE mechanisms should be tailored to the device architecture. Future research on the nuances of the SiO2 ALE mechanism is essential to further enhance the precision and efficiency of these processes in semiconductor fabrication.