ABSTRACT
Quartz crystals are the most widely used material in resonant sensors, owing to their excellent piezoelectric and mechanical properties. With the development of portable and wearable devices, higher processing efficiency and geometrical precision are required. Wet etching has been proven to be the most efficient etching method for large-scale production of quartz devices, and many wet etching approaches have been developed over the years. However, until now, there has been no systematic review of quartz crystal etching in liquid phase environments. Therefore, this article provides a comprehensive review of the development of wet etching processes and the achievements of the latest research in this field, covering conventional wet etching, additive etching, laser-induced backside wet etching, electrochemical etching, and electrochemical discharge machining. For each technique, a brief overview of its characteristics is provided, associated problems are described, and possible solutions are discussed. This review should provide an essential reference and guidance for the future development of processing strategies for the manufacture of quartz crystal devices.
I. INTRODUCTION
Quartz crystals are among of the most widely used piezoelectric materials and are critical ingredients in many actuators and sensors, owing to their excellent properties such as low coefficient of thermal expansion, corrosion resistance, chemical stability, and electrical insulation.These characteristics allow quartz crystal devices to operate successfully in harsh environments, such as searing heat, freezing cold, and crushing pressure.
Quartz crystals are commonly used in the manufacture of resonators, quartz crystal microbalances (QCMs), and inertial devices. Quartz resonators have become indispensable components of many electronic devices. In addition to conventional quartz resonators with frequencies in the tens of megahertz range, ultrahigh-frequency quartz resonators with fundamental frequencies in the hundreds of megahertz and gigahertz ranges have been reported.QCMs are based on resonators whose resonant frequency varies with external conditions, such as surface deposition mass, humidity, and ambient gas density. They are therefore extensively used for environmental signal detection, chemical reaction supervision, and gas detection, and in biomedicine and device manufacturing.Quartz crystals are also commonly used in resonant accelerometers and gyroscopes,which exploit their characteristics such as simple fabrication, excellent temperature stability, low power consumption, high quality factor, and low cost. Furthermore, quartz crystals also commonly serve as contact scanning microprobes,such as atomic force microscopy (AFM) probes.
Although wet etching has been developed over many years and improvements have been made recently, there has yet to be a systematic review of the etching of quartz in a liquid phase environment. At present, with the development of wearable portable electronic devices, there is increasing demand for miniaturized, high-precision, and low-power-consumption quartz crystal devices. Therefore, it is appropriate to summarize and analyze the results of research carried out over the past few decades on quartz crystal etching in liquid-phase environments, describe new fabrication processes, and provide a necessary reference and guidance for the future development of manufacturing processes for quartz crystal devices. In this article, we will focus on the effects of etching on quartz crystals in a liquid phase environment and present a comprehensive review of the reaction mechanisms involved in the various etching methods. The review first introduces and discusses the basic principles and characteristics of quartz crystal etching in conventional etching solutions. Then, the details and results of research into other etching processes in a liquid phase environment are discussed, such as additive etching, laser-induced backside wet etching (LIBWE), electrochemical etching, and electrochemical discharge machining (ECDM). This review presents the underlying rationale for each method and illustrates its development and current progress. Some considerations regarding the advantages and disadvantages of each method are also presented. Finally, the current problems that need to be solved with regard to quartz crystal fabrication are discussed, as well as some possible solutions and prospects for further exploration.
II. QUARTZ CRYSTALS
Quartz crystals with chemical composition SiO2 occur widely in nature. In terms of its crystal structure, quartz belongs to the tripartite crystal system. In a quartz crystal, each silicon atom is surrounded tetrahedrally by four O atoms, with the shape of this tetrahedron departing slightly from that of a perfect tetrahedron owing to the anisotropy of the quartz crystal. Figure 1(a) shows the atomic arrangement of a typical crystal surface of a quartz crystal.Zig-zag –Si–O–Si–O– chains form an interleaved connected structure, which results in loss-free piezoelectricity.There are two chiral forms, namely, normal α-quartz and hightemperature β-quartz, which crystallize respectively in a trigonal crystal system [Fig. 1(b)] and a six-axis crystal system [Fig. 1(c)]. β-quartz can form directly from high-temperature gas or solution. It always undergoes a transition to α-quartz on cooling, and, conversely, α-quartz, when heated above the transition temperature, is transformed into β-quartz. The transformation from α-quartz to β- quartz takes place at 573℃.When α-quartz is transformed into β-quartz, there is a significant change in volume owing to the relatively small rotation of the crystal structure without any change in atomic connections. As β-quartz exists only at high temperatures and α-quartz is the form most commonly used to make devices, this review focuses only on the properties and etching of α-quartz.
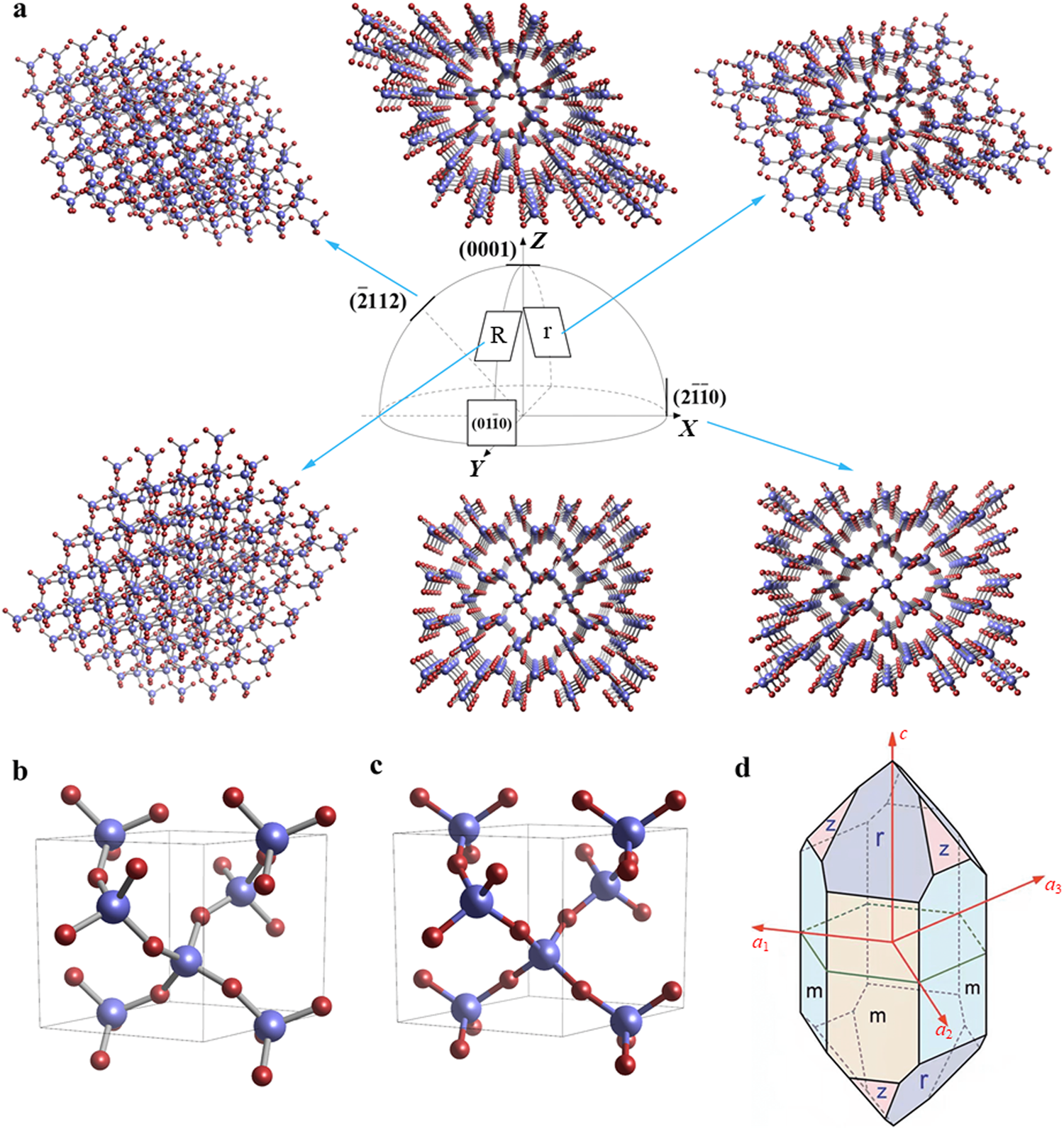
Fig1
Interestingly, according to Ernsberger’s theory, (2112), r, R, and m crystalline planes are unable to react with HF etchant. However, Cheng et al. reported experiments showed that r planes have higher etching rates, while m and R planes have lower etching rates. Thus, the dangling bond theory can only explain the etching rate phenomenon for a specific crystal plane and has limitations in explaining the anisotropic etching of quartz. In addition, the etching rate of quartz is considered to be related to the activation energy. In 1961, Schwartz and Robbins suggested that there was a correspondence between the surface activation energy of silicon single crystals and the rate of etching of the crystal surface. Inspired by this, Zhang experimentally confirmed that a similar relationship exists in quartz crystals. The higher the concentration of high-activationenergy atoms on a crystal surface, the higher is the surface activation energy and the lower is the etching rate. This means that the atomic composition and structural arrangement of the quartz crystal surface can determine the surface activation energy and thus influence the etching rate. For example, (0001), (2112) and (0111) planes contain atoms with high etching rate and have low surface activation energies. At the same time, the (2112), (0110), and (0111) crystalline planes with a lower etching rate have surface atoms with a higher surface activation energy. This theory complements the dangling bond theory in terms of microscopic atomic activation energies.
Conventional chemical etching uses liquid chemicals to remove material and thereby fabricate a device structure. Etchant is brought into direct contact with the surface of the etched material without the use of auxiliary means. This is the most popular fabrication method in both scientific research and industrial production, on account of its simplicity and convenience. Since the first study of the etching of quartz in 1889,there have been numerous investigations of the many factors that play a crucial role in the etching process, including etchant concentration, temperature, and dopants.However, despite these extensive studies and the knowledge that they have provided, the conventional chemical etching process still faces a number of challenges, especially in micro–nano manufacturing. On the one hand, etched cross morphologies are irregular because of the complex crystallographic orientation of the quartz crystal, which induces differences in etching rate and uniformity. On the other hand, there are a variety of influences on etching behavior. Not only can etchant concentration and temperature, among other factors, make a difference, but even the width and depth of etched grooves can affect the final results of the process. Therefore, the etched cross morphology has been widely explored, and this section discusses the common factors which affect the conventional wet etching process.
It can be seen that the fluoride-based solutions consist mainly of − , HF2 − , and un-ionized HF molecules. It has also been suggested that (HF)2 or (HF)t molecules are produced in solution owing to hydrogen bonding of HF molecules.During the etching process, H+ is mainly adsorbed at the solid–liquid interface as a catalyst. Polymeric ions (HF)tF − are also produced in solution when the HF concentration is higher than 1 mol/l.When the HF concentration is exceeds 1.4 mol/l, the solution is dominated by HF2, (HF)t, and HF molecules. The curves in Fig. 2(a) show the [(HF)2]–[HF]t and etching rate (v)–[HF]t relationship.67 It can be seen that the etching rate and the (HF)2 concentration both increase with increasing of HF concentration, but the [HF]–[HF]t curve does not completely overlap with the [(HF)2]–[HF]t and the v–[HF]t curves. Therefore, the reaction rate is mainly controlled by the concentration of (HF)2.
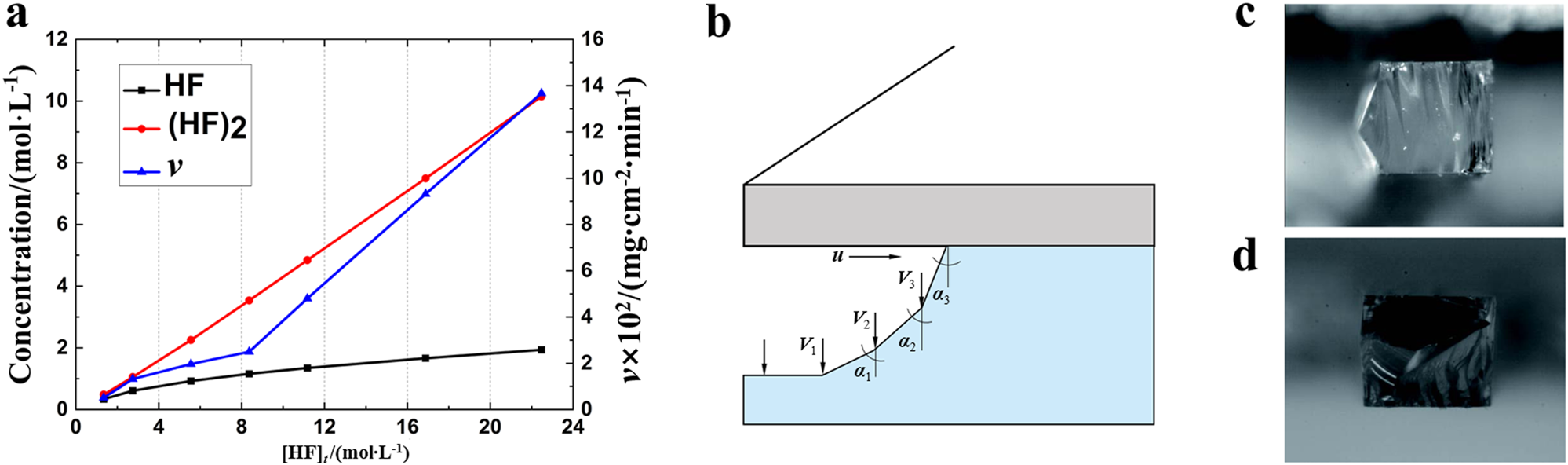
Fig2
Masruroh et al.74 etched quartz crystals using three different solutions with KOH mass fractions of 25%, 30%, and 35% at a temperature of 80 ○C and atmospheric pressure. Their experiments showed that even the fastest etching condition with a mass fraction of 35% KOH could only reach an etching rate of 0.354 μm/h. Figures 3(a)–3(c) show the etched and unetched surfaces. It can be observed that the surface roughness after etching with KOH solution is much better than before etching, with 30% KOH giving the best improvement, with a surface roughness of 249 nm, although this is still not sufficiently smooth when compared with etching by fluoride solution.
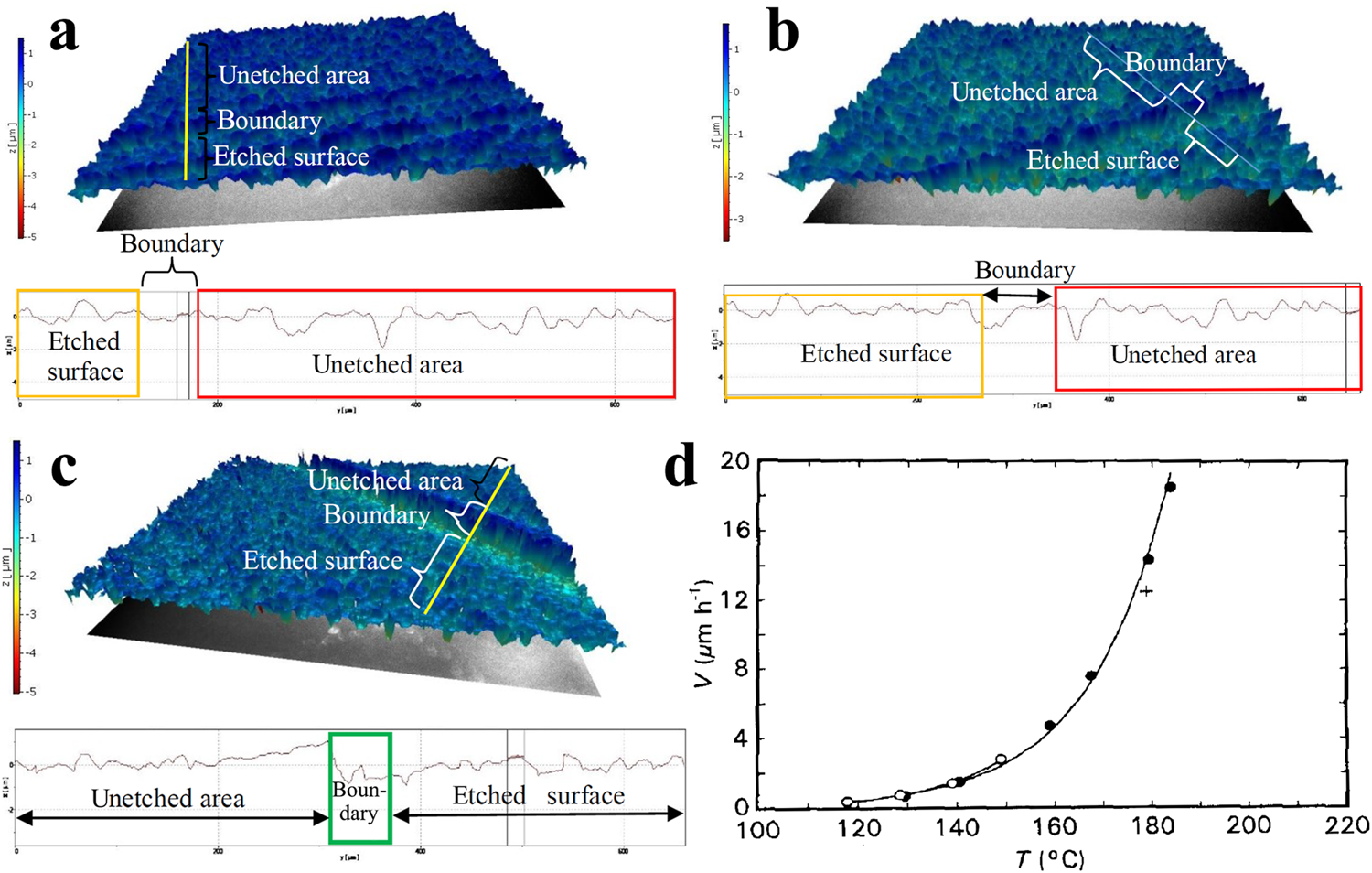
Fig3
In summary, the use of additives in the etching of quartz crystals can effectively compensate for the shortcomings of conventional etchants. It also plays an outstanding role in optimizing the etching rate and improving the etched profile of quartz crystals. However, at present, research into the use of additives in quartz etching is still in its infancy. There remains a lack of clarity regarding the mechanisms by which some additives affect the etching process, and the effects of new types of additives, especially those including long-chain molecules, on the etching process is also worth further investigation.
上一篇: 微流控芯片中微气泡聚集的表征
下一篇: CuS多孔硅异质结的光学特性