Abstract
Thin wafer have become a basic need for a wide variety of new microelectronic products. Wafers that have been thinned using wet etch process on the backside have less stress compared with standard mechanical back grinding. Isotropic wet etching of silicon is typically done with a mixture of nitric and hydrofluoric acids. As the silicon is etched and incorporated in the etching solution the etch rate will decrease with time. This variation has been modeled. The focus of this paper is to compare the process control technique for maintaining a consistent etch rate as a function of time and wafer processed.
Introduction
Micro Electro Mechanical Systems (MEMS) is an integration of mechanical elements and electronic circuit on a common substrate through the use of microfabrication technique to achieve high performance devices with dimensions ranging from less than a micron to several microns. Most of the MEMS devices are currently based on silicon because of the available surface machining technology. Silicon, a MEMS material, has been chosen for investigation with particular emphasis in etching. Etching technology in thin film process plays an important role in the semiconductor industry. Isotopic/anisotropic etching of silicon is used to obtain varied microstructures. Anisotropic etching of silicon is extensively done by taking KOH solution as etchant. In semiconductor processing because of their low cost, high throughput, and excellent selectivity. Important progress in the fabrication of microelectrical structure with integrated circuits has been achieved by many researchers using KOH wet etching. Fabrication of UMOS transistors on Si (111) wafers for high power and high current densities has been achieved by applying KOH anisotropic wet etching to the silicon substrate. Other applications include the fabrication of VMOSFETs, radio frequency amplifiers, power supplies and microcomputers. Using its selective and anisotropic etching properties, KOH has been applied to yield devices snel as field emission devices, optical waveguides, pressure sensors and ink nozzles.
Experimental Procedure
The development of method for the production of miniaturized mechanical components and devices with Si is a natural outgrowth of Si surface machining methods have been developed for the production of microcircuits. Proceed towards this direction, we prepare fresh KOH solution by weighing 1 part KOH pellets into a plastic beaker and then add 2 parts of DI water. As an example, use 100 g KOH with 200 ml water. Mix on warm surface until KOH has dissolved. Add 40 ml of isopropyl alcohol to the solution. The isopropyl alcohol increases the anisotropy in etching. The KOH etch requires a “hard mask” of silicon dioxide or silicon nitride (nitride is preferred since oxide is slowly etched by KOH). The details on making a hard mask can be found elsewhere. The basic approach is as follows. Start with silicon (100) polished wafer. Clean wafers and pattern with photoresist. Use the reactive ion etches (RIE) system to etch the exposed oxide or nitride surface, for oxides: CHF3 and O2 or CF4 and O2 . Etch until the silicon is exposed (shiny); typically 5 minutes per 1000 Å film. Rinse the wafer with acetone to remove the remaining photoresist. Rinse with DI water, and then blow dry. Put KOH solution in glass container and warm to 80° C on a hot plate. If desired, use a mixer to agitate the solution. Place patterned wafer (with patterned hard mask) in the KOH solution. The KOH will bubble at the exposed silicon sites while etching occurs. The etch rate for 30% KOH at 80°C should be about 1 micron/minute. Rinse all labware three times in clean water. In very small amounts (less than 30 ml): Dilute the KOH with cold water, then neutralize with a small amount of HCl. If the pH is below 12.5, pour the solution down the drain, flushing with plenty of cold water.
The wafer used in this study was thermally bonded silicon on insulator (SOI) wafer with (100) orientation. The thickness of the top Si, buried SiO2 layer and bottom Si. The wafers are of p-type. Only the top Si layer was etched by the KOH solution. First of all, the SOI wafers are prepared with standard RCA cleaning. A 450 Å layer of Si3N4 , which acts as a KOH etching mask, deposited on each wafer using low pressure chemical vapor deposition (LPCVD). Oxide can be used as an etch mask for short periods in the KOH solution. For long periods, nitride is a better etch mask as it etches more slowly. The SOI wafer then cut into 5mm × 5mm pieces. With the diced pieces, positive photoresist, will be used to pattern the Si3 N4 for the KOH etching mask. The nitride etched with the exposed portion of the top Si on each SOI wafer and etched in KOH solutions of varying temperature. To assure the samples free of particulate and other airborne contaminants, the experiment was conduced in a clean room environment.
Results and Discussion
Silicon Etching
The chemistry most commonly used for isotropic wet etching of silicon is a combination of nitric acid and hydrofluoric acid. It is very often referred to as the HNA system (HF:Nitric:Acetic) with Acetic acid is added as a buffer for wet bench application. The nitric acid acts as an oxidizer to convert the surface into silicon dioxide and then the HF etches (dissolves) the oxide. The reaction proceeds as shown below and has been well documented in the literature.
As we have seen, HNO3 oxidizes Si, and HF etches the SiO2 hereby formed. High HF: HNO3 ratios promote rate-limited etching (strong temperature dependency of the etch rate) of Si via the oxidation 1-3, while low HF: HNO3 ratios promote diffusionlimited etching (lower temperature dependency of the etch rate) via step (4). HNO3 free HF etches do not attack Si. The SiO2 etch rate is determined by the HF-concentration, since the oxidation does not account. Compared to thermal oxide, deposited (e. g. CVD) SiO2 has a higher etch rate due to its porosity; wet oxide a slightly higher etch rate than dry oxide for the same reason. An accurate control of the etch rate requires a temperature control within ± 0.5°C. Dilution with acidic acid improves wetting of the hydrophobic Si surface and thus increases and homogenizes the etch rate. Doped (n- and p-type) silicon as well as phosphorusdoped SiO2 etches faster than undoped Si or SiO2.
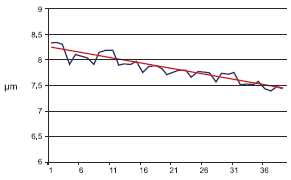
Fig1
Using this equation and comparing with the data we have obtained from the experiment are looking to be consistent. It should be noted that the above equation and resulting decrease in etch rate is dependent upon the wafer size (area of silicon being etched). For this investigation, wafer size was 150mm. For larger wafers the decrease in etch rate will be greater. To eliminate the variation in etch rate as wafers are processed we have two obvious options: increase the etching time or spike/replenish the active chemicals. Using the model and data we have acquired the following comparison. The data and model indicates that our etch rate is decreasing by 2.5% every 10 wafers. This translates into a 1 second increase in etch time after 4 wafers. The resulting etch rate is more consistent however the etch time and therefore tool throughput decreases. For a tool with an initial throughput of 25 wph, this would decrease to 15 wph after 16 hours (and 400 wafers) of processing.
While changeover from etch to rinse one should stop collecting the chemical in order to avoid the addition of water into the chemistry. The amount of chemical lost during this time (less than a second) is approximately 30ml at the flow rate we are using. Therefore, after processing 400 wafers we would have depleted the chemical supply by 12 liters and it would need to be refilled. Also at some point the amount of silicon in solution will be at a maximum and the chemistry will need to be replaced. Another way to maintain a constant etch rate is to either spike the chemical mixture with the active ingredient (HF) or to continuously remove and replenish the chemical solution or some combination of these. Our calculations are based on the 25 liter volume within our recirculating chemical system. The ratio for filling the system with chemicals based on the chemical mix of 1:6:1:2 (Hydrofluoric, Nitric, Sulfuric and Phosphoric). Option one is to replace (remove and add) a percentage of the solution for every wafer. A second option is to spike with HF based on 10% of the initial solution volume and the 0.25% decrease in etch rate observed. However, this could not be done indefinitely due to some minimal loss of chemical during the switchover to water rinsing. The spiking with HF can be combined with adding enough of the chemical mixture to make up for the amount lost during changeover to the rinse cycle. Although both techniques will maintain a more stable etch rate, increasing the etch time will decrease the wafer throughput and require periodic shutdown for chemical disposal and refill resulting in lower system utilization. Chemical replenishment will maintain the wafer throughput; provide continuous chemical disposal and replenishment resulting in higher system utilization and overall lower cost of ownership.
Conclusion
There are many wet-chemical etch recipes known for etching silicon. These processes are used for a variety of applications including micromachining, cleaning, and defect delineation. The detailed behaviour and rate of the etchant will vary between laboratory environments and exact processes. As silicon wafers are etched a decrease in etch rate is observed. Spiking with HF provides a means to replenish the active component. At the same time, silicon is building up in the solution in the form of hexafluorosilicic acid. The wafer size is determine the spiking, removal and fresh make-up quantities for a stable equilibrium to be reached where the solution is self-replacing. This is the lowest cost of ownership in terms of chemical costs and system down time and will result in a constant etch rate with time.
上一篇: 化合物半导体的选择性蚀刻
下一篇: 射频微系统封装工艺技术