A surface chemical reaction model of silicon dioxide fifilm etching by hydrogen flfluoride aqueous solution using a single wafer wet etcher was numerically evaluated taking into account the Langmuir-type rate theory and the transport phenomena. The surface reaction process was assumed to consist of three steps, such as (i) hydrogen flfluoride adsorption at the silicon dioxide surface, (ii) chemical reaction of silicon dioxide with hydrogen flfluoride and (iii) desorption of the by-product from the surface. The rate constants determined by calculation which could reproduce the silicon dioxide etching rate obtained by experiment. The rate limiting step was additionally evaluated.
A silicon-on-insulator (SOI) wafer is currently a very important material, which is prepared by the wafer-bonding technique using two silicon wafers.1–4 This production process requires a very clean and flflat surface of a silicon dioxide fifilm formed on the silicon wafer sur face. In order to effectively improve the silicon dioxide fifilm thickness uniformity after the oxidation, the single wafer wet etching method5–9 is a useful technique. Its further advance should be supported by theoretical calculation. Thus, the numerical calculation model for the silicon dioxide fifilm etching using the single wafer wet etcher was developed in our previous studies.6,9 First, the entire water motion on the rotating wafer was obtained by the water flflow visualization, and was evaluated to show that the velocity and the layer thickness of water could be reproduced by the numerical calculation.6 Next, the silicon dioxide etching rate by hydrogen flfluoride aqueous solution could be obtained assuming the simple rate equation.9 This model could simultaneously clarify the role of a swinging nozzle. However, a small discrepancy in the etching rate between the calculation and the measurement remained. In order to obtain an accurate and practical calculation model, the surface chemical reaction process and its rate, such as the Langmuir model, should be appropriately described like chemical vapor deposition.10–12 In this study, the practical numerical calculation model was thus developed and validated for the silicon dioxide fifilm etching by hydrogen flfluoride aqueous solution, using the single wafer wet etcher.
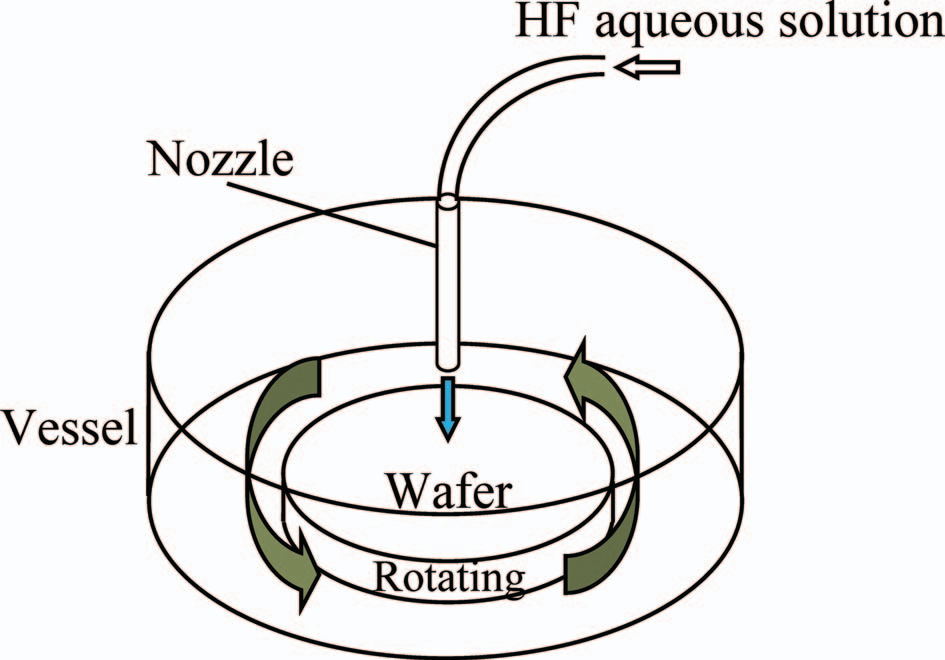
Fig. 1 shows the single-wafer wet etcher used in this study. This etcher has a 200-mm diameter wafer rotating at the rate of 100–1400 rpm in a cylindrically-shaped vessel. The hydrogen flfluoride aqueous solution (3%) was injected downward from the 4-mm diameter nozzle normal to the wafer surface at the flflow rate of 1 L/min for 1 min. After the injection, the hydrogen flfluoride aqueous solution was transported along the rotating wafer surface from the injected position to the wafer edge, then fifinally spun off from the wafer edge to the outside. The entire process was performed at room temperature, following industrial conditions. Because the hydrogen flfluoride concentration used in this study is very low, the reaction heat produced at the wafer surface is very low. Additionally, the fast liquid flflow maintains the surface temperature at the inlet temperature. Thus, we ignored the temperature change due to the reaction heat. The silicon wafer used in this study had a 100-nm thick silicon dioxide fifilm, formed by oxidation. The silicon dioxide fifilm thickness was measured using an ellipsometer (M8300XMP, Nanometrics Japan, Inc., Tokyo) before and after etching, in order to obtain the etching rate.
上一篇: 单晶刀清洗过程:分散现象对冲洗时间的影响
下一篇: 氮化栅氧化物的表面制备挑战