The effect of various metal contaminants on the thin gate oxide integrity is investigated and a classification is made according to their final position in the structure. A simplified cleaning strategy Is presented which is highly performant and at the same time cost-effective and has less environmental impact than the traditional cleaning sequences. Finally, a novel environmentally friendly ozone/DI-water process for the removal of photoresist and organic post-etch residues is proposed.
In view of the important effect of contamination on the device performance and process yield, it can be easily understood that cleaning is the most frequently repeated step in IC-production. A relatively large volume of DI-water and chemicals is consumed in these steps, which induces an important production cost and causes serious environmental concerns. Therefore, over the last years a lot of research effort was directed to the development of cleaning techniques that are more performant, cost-effective and have a lower environmental impact.
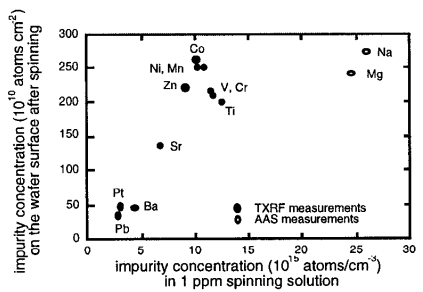
The behaviour of several contaminants which are commonly found in cleanroom materials (Na, Mg, Cr, Zn, Ni, V, Mn) or may be used in future dielectrics (Ti, Sr, Ba, Pt, CO, Pb) was investigated. The silicon wafers were cleaned to obtain a contamination-free reference hydrophilic surface. The contaminants were applied by spinning an acid solution with pH=O. 1 containing 1 ppm of the contaminant under investigation. Due to the varying atomic masses of the elements, the impurity concentrations varied over an order of magnitude (fig.1).
After contamination, 4.5 nm oxides were grown in a dry 0, ambient at 800°C. The contaminants used in this study do not lead to a significant difference in the final oxide thickness (fig.2), except for Na which is known to enhance the formation of crystalline SiO, [ l]. In most cases the contamination level decreases after oxidation (fig.3). In the case of transition metals, a trend towards higher contamination loss is seen for elements with larger atomic mass. This can be partly explained by the higher diffusivities and solubilities of these elements in silicon [2]. Na, Zn and Pb partly evaporate during oxidation. No loss for Mg is observed, probably due to the formation of Mg,SiO, or MgSiO, silicates [3].
上一篇: 蚀刻过程中砷在GaAs上的形成
下一篇: GaAs 和GaN 的湿热氧化