1. Introduction
The chapter is devoted to the thermal wet oxidation of AIIIBV semiconductor compounds, mainly to gallium arsenide and gallium nitride. It has been divided into several topics, containing of monoclinic gallium oxide1 -Ga2O3 properties data, techniques of oxide fabrication and application description. In the first part, properties of mentioned semiconductor’s oxides are characterized. Then methods of manufacturing with a special attention for wet thermal oxidation are described. After that, applications of gallium oxide structures in electronics are given. It focuses also on the semiconductor structures dedicated for gas sensors application while gallium oxide layers improve significantly the most critical parameters of the detector compared to those containing of e.g. SnO2. AIIIBV and AIIIN semiconductors compounds are wide known as materials for optoelectronics devices. They are used often also to the construction of high temperature and microwave devices or chemical gas sensors. In these applications dielectric layers are necessary. There is a possibility of using their own oxides – Ga2O3 gives a chance to manufacture many different devices – MOS structures (Metal-Oxide-Semiconductor). It can be MOS capacitors, power Metal Oxide Semiconductor Field Effect Transistors (MOSFETs), high mobility GaAs MOSFETs or gate turn-off thyristors and, probably, CMOS applications (Pearton et al., 1999; Wu et al., 2003). The MOS-gate version of the HEMT has significantly better thermal stability than a metal-gate structure and is well suited to gas sensing
2. Properties
of -Ga2O3 Gallium oxide -Ga2O3 is a wide band gap material that ensures deep-UV transparency. Appropriately doped could reach conductive properties thus is included to the TCO’s (transparent conductive oxides) materials like ITO or ZnO which are the state-of-the-art materials in optoelectronics. Gallium oxide occurs in various structures like , , , , types (Kim & Kim, 2000). Among many polymorphs, monoclinic -Ga2O3 is considered to be the equilibrium phase (Battiston et al., 1996; Chen et al., 2000; V´llora et al., 2004). It is stable thermally and chemically(Battiston et al., 1996; V´llora et al., 2004). The thermal stability of -Ga2O3 reaches nearly melting point reported as 1740 C (Orita et al., 2004) and 1807 C (Tomm et al., 2000) or 2000 K (V´llora et al., 2004) what determines also possibility of working at high temperature. - Ga2O3 in monoclinic structure has a elemental unit dimensions as follows: a=12.214 Å, b=3.0371 Å, c=5.7981 Å and =103.83 (Tomm et al., 2000) or a=12.23 Å, b=3.04 Å, c=5.8 Å and =103.7 (V´llora et al., 2004). Cleavage along (100) plane (Tomm et al., 2000; Ueda a et al., 1997; V´llora et al., 2004) and (001) (V´llora et al., 2004) are highly preferred. The space group of -Ga2O3 is C2/m (C3 2h) where GaO6 share octahedral sites along b and are connected by GaO4 tertrahedra thus anisotropy of optical as well as electrical properties is expected depending on the direction to the chains – perpendicular or parallel (Ueda b et al., 1997). The -Ga2O3 unit cell along b, c and a-axis could be found in (V´llora et al., 2004).
2.1 Electrical properties At room temperature -Ga2O3 is an insulating material, above 500 C has a semiconductor properties (Fleischer & Meixner, 1993; Battiston et al., 1996; Frank et al., 1996; Orita et al., 2004). Although electrically conductive crystals of -Ga2O3 have been also reported, see Table 1 (V´llora et al., 2004)
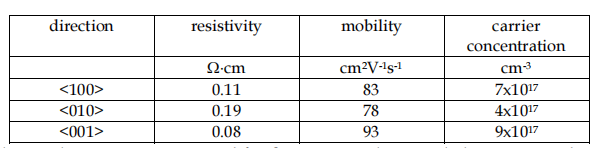
Table 1. Electrical properties measured for β-Ga2O3 single crystal along certain direction
上一篇: 硅加工的高效清洁