ABSTRACT
The ~4Cu monitor method was used to study the impact of DI H20 rinses on the cleaning efficiency of fluorinated silicon surfaces. Omission of the DI H20 rinse between oxide removal by HF/H20 vapor and the cleaning solutin improves the cleaning efficiencies considerably on p-doped and slightly on n-doped silicon wafers in comparison to the identical process with a DI H20 rinse.
Efficient silicon surface cleaning is required for obtaining high product yields and reliability for semiconductor devices. It has been shown that peroxide solutions are capable of removing organic and metallic contamination from silicon (1, 2). The presence of a native oxide layer on the silicon surface is suspected to inhibit the surface cleaning (3). Cleaning sequences have been proposed that eliminate this native oxide layer, prior to cleaning, by immersing silicon wafers into HF-solutions (3, 4). These HF immersions are followed by a DI H20 rinse in most semiconductor processing facilities. The interaction between fluorinated silicon surfaces and DI H20 has been the object for several studies (5-7). Surface analysis of fluorinated silicon surfaces after DI H20 rinses revealed a significant carbon contamination (6, 7). The effect of such a contamination on the silicon surface cleaning efficiency has been suspected, but never measured in any quantitative terms (8). In this paper, we investigate the impact of DI H20 rinses on silicon surface cleaning by comparing the cleaning efficiency with and without DI H20 rinse after an oxide removal. After wafer withdrawal from conventional liquid HF solutions, substantial amounts of HF can adhere to the wafer surface and an immediate dip into an oxidizing cleaning solution such as a peroxide solution would form a silicon etch, which can cause severe silicon surface erosion. Only very small amounts of HF should be present in the peroxide solution in order to prevent any surface damage (1). Such small amounts of HF can be easily introduced by utilizing HF/H20 vapor mixtures formed over a liquid HF-solution at room temperature (9).
The cleaning sequence studies were carried out with ammonia-peroxide (1 part NH4OH, 1 part H202, 5 parts DI H20) and hydrochloric acid-peroxide (1 part HC1, 1 part H~O, 5 parts DI H20) solutions (1). Parts of these cleaning solutions were also investigated such as H20~ (1 part H20,2, 5 parts DI H20) and HCI (l part HC1, 5 parts DI H20) solutions. All wafers were immersed for 5 min into the cleaning solutions, which were kept at a temperature range between 60 ~ and 70~ and agitated ultrasonically. This temperature range was adopted instead of the 90~ temperature employed previously (1) to retard peroxide depletion occurring rapidly at elevated temperatures. After completion of the cleaning, all wafers were rinsed for 5 min in 14-16 MI2 DI H20 and then spun dry in N2. Electronic grade chemicals were used in this study. DI HeO was" supplied by recycling loop consisting of a prefilter (Millipore CD15), a charcoal filter (Millipore CDFC 02203), an ion exchanger column (Millipore CDMB 02202), and a final filter (Millipore HA 0.45 #m). A schematic drawing of the HF/H20 vapor etching method is shown in Fig. 1. Liquid 49% HF covers the bottom of a plastic container. The wafer basket is positioned 2 in. above the liquid HF surface during the HF/H20 exposure. The plastic cover is required to generate sufficient HF/H20 vapor pressure in the container. At room temperature, a 1 min exposure removes approximately 100 nm of thermally grown SiO2 on silicon wafers. In the'presence of moisture, the following chemical reaction occurs at the silicon surface (9, 10) SiO2 + 3(HF)2~ H2SiF6 + 2H20 The hexafiuosilicic acid (H2SiF6), which is formed at the silicon surface, is water soluble (10) and can therefore be removed by a successive aqueous rinse.
Results and Discussions Tables I through IV list the various cleaning solutions used and their cleaning efficiency including the spread between average value and the measured
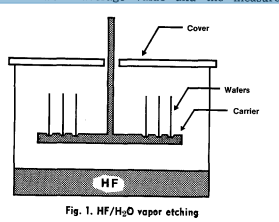
Fig. I. HF/H~O vapor etching
Summary DI H20 rinses of bare fluorinated silicon surfaces form a contamination layer that inhibits silicon surface cleaning. The elimination of DI H20 rinses between HF/H20 vapor exposure and cleaning solution immersion produces high cleaning efficiencies and will not introduce any silicon surface attack as long as only small amounts of HF are deposited on the silicon surface. The removal of native oxide layers on silicon surfaces is essential for obtaining a high cleaning efficiency. Contamination layers, formed by DI H20 rinses of fluorinated silicon surfaces, will not inhibit the surface cleaning, if the contamination layer is exposed to HF/H20 vapor followed by an immediate cleaning solution immersion.Manuscript submitted May 21, 1981; revised manuscript received Oct. 9, 1981. Any discussion of this paper will appear in a Discussion Section to be published in the December 1982 JOVRNAL. All discussions for the December 1982 Discussion Section should be submitted by Aug
上一篇: 前端清洗工艺中的硅化物残留
下一篇: 旋涂金属氧化物及其在下一代光刻中的应用