Abstract
Silicon is by far the most important semiconductor material in the microelectronic industry mostly due to the high quality of the Si/SiO2 interface. Consequently, applications requiring chemical functionalization of Si substrates have focused on molecular grafting of SiO2 surfaces. Unfortunately, there are practical problems affecting homogeneity and stability of many organic layers grafted on silicon oxide (SiO2), such as silanes and phosphonates, related to polymerization and hydrolysis of Si-O-Si and Si-O-P bonds. These issues have stimulated efforts in grafting functional molecules on oxide-free Si surfaces, mostly with wet chemical processes. This review focuses therefore directly on wet-chemical surface functionalization of oxide-free Si surfaces, starting from H-terminated Si surfaces. The main preparation methods of oxide-free Hterminated Si and their stability are first summarized. Functionalization is then classified into indirect substitution of H-termination by functional organic molecules, such as hydrosilylation, and direct substitution by other atoms (e.g. halogens) or small functional groups (e.g. OH, NH2) that can be used for further reaction. An emphasis is placed on a recently discovered method to produce a nanopattern of functional groups on otherwise oxide-free, H-terminated and atomically flat Si(111) surfaces. Such model surfaces are particularly interesting because they make it possible to derive fundamental knowledge of surface chemical reactions.
Keywords Silicon surfaces, hydrogen-termination, organic functionalization, self assembled monolayers, surface activation, nanopatterning
Abbreviations Si, silicon; SiO2, silicon oxide; SAM, self-assembled monolayer; XPS, X-ray photoelectron spectroscopy; FT-IR, Fourier transform infrared; AFM, atomic force microscopy; NN, nearest neighbor; NNN, next nearest neighbor; RT, room temperature; TFT, thin film transistor; ALD, atomic layer deposition; MPA, methylphosphonic acid; ODPA, octadecylphosphonic acid; DFT, density functional theory; KMC, kinetic Monte Carlo; ML, monolayer; H, hydrogen; T-BAG, tethering by aggregation and growth; OH, hydroxyl; UHV, ultra-high vacuum; MOF, Metal Organic Frameworks; SURMOF, surface Metal Organic Frameworks; LBL, layer-by-layer; PL, photoluminescence; F, fluorine;
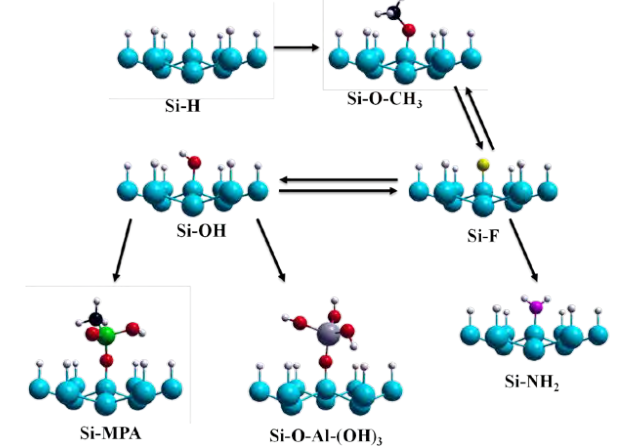
Figure 1: Overview of wet chemical reactions associated with nanopatterning: First,
3.2.2. Amino-termination Amines are ubiquitous in biology.99 Protonated amino groups (-NH3 + ) are the most common positively charged moieties in proteins, specifically in the amino acid lysine. The anionic 10 polymer DNA is typically bound to various amine-rich proteins. Additionally, the terminal charged primary ammonium on lysine forms salt bridges with carboxylate groups of other amino acids in polypeptides, which is one of the primary factors of the three-dimensional structures in proteins. There has recently been some progress made in obtaining amino functionality based on the reactions of ammonia with a clean Si surface in UHV environments. For example, ammonia reaction with clean Si(100)-2x1 surfaces has been shown experimentally and theoretically to produce Si-NH2 and Si-H species in UHV conditions at RT. 100-111 Upon annealing to 230 ºC, SiNH2 species start decomposing, resulting in the insertion of N atom to the neighboring Si-Si dimer and the formation of (Si)2=N-H. However, the clean Si(100)-2x1 surface appears unstable (i.e. suffers oxidation) outside UHV conditions. So far the ability to prepare oxygen- and carbonfree Si surfaces uniformly terminated with –NHx functionalities by wet chemistry methods has remained elusive. On the other hand, high quality H-terminated Si surfaces can be prepared by wet chemistry methods and are stable for some time under ambient conditions. Recent detailed vibrational and mechanistic computational studies by Dai et al. indicated that H-terminated Si(111) surfaces can react with gas-phase ammonia112. The thermal reaction becomes rather complex at elevated temperatures due to N insertion into Si-Si bonds. This reaction was shown to depend on surface morphology, with ammonia reacting first with steps then atomically flat H-Si(111) terraces. Exposing H-terminated Si(111) surfaces to gas phase ammonia led to the formation of Si-NH2 at first at 350ºC, then with temperature increasing to 400ºC the N atom inserted into Si-Si framework to produce a (Nx)Si-NHy layer. Recently, Tian et al. suggested that immersion of Cl-terminated Si surfaces in an ammoniasaturated THF solution at RT leads to amine attachment on Cl-terminated surfaces. This work raises interesting questions on the nature (NH vs. NH2) and structure (Si-NHx vs. Si-NH-Si) of the bonded NHx species. 113,114 We note however that experimental evidence for amine termination of Cl/Si surfaces in NH3 vapor has been questioned by Lange et al. 115. Total-energy calculations show that the primary amine formation as suggested by Finstad et al.116 is endothermic. Interestingly, the formation of an ionic complex at the surface was found to be more stable than the desorption of HCl (see figure 8).
上一篇: 外延晶圆中金属污染的表征
下一篇: 芯片制造中的电化学加工技术:挑战与机遇