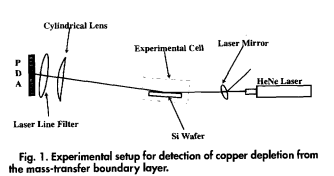
An optical technique based on simple absorption spectroscopy has been demonstrated for monitoring metal contaminant deposition from aqueous processing solutions widely employed during microelectronics fabrication. Cu deposition from 0.15 and 0.25% HF solutions contaminated with 3.5 ppm Cu has been observed as a decrease in the absorption of a HeNe laser reflected at glancing incidence from a Si wafer. This is caused by the depletion of Cu2 from the mass-transfer boundary layer, providing direct evidence that Cu deposition is rate-limited by diffusion. This technique allows deter- mination of which metallic species can deposit onto Si wafers in diffusion-limited processes during various aqueous pro- cessing steps in microelectronics manufacture. In addition, Cu deposition is seen to coincide with the completion of Si07 dissolution, confirming that Cu2 is reduced by an electroless process involving simultaneous Si oxidation. The decrease in absorption is consistent with a mass-transfer boundary layer thickness of about 335(20) lim. The optical absorption returns to its original value about 2 mm after the onset of deposition, consistent with diffusion-limited deposition of one monolayer of Cu, which is followed by a transition to a slowe surface rate-limited process.
Aqueous processing of semiconductors has been widely employed for removing residual metals, organics, and particles from the silicon substrates on which microelectronic devices are fabricated. A typical cleaning sequence may include a HF etch, immersion in SC-i and SC-2 cleaning solutions, and deionized water rinses. The purpose of the HF etch is to remove the native oxide (5i03), which often has significant contamination arising from cutting and polishing as well as transport and storage. Various formulations exist for the SC-i (H30-H707-NH,) and SC-2 (H,OH30,-HC1) solutions, with recent trends suggesting that more dilute solutions will be employed in the future.' The oxidizing action of H202 decomposes organics and other species, while the strongly complexing Cl- solvates metal contaminants.2 However, the degree of surface metal contamination depends strongly on the effectiveness of wet cleaning and the purity of the reagents employed. Since the direction of equilibrium can lie in either direction, each of these aqueous solutions may either deposit or dissolve a trace metal contaminant. Thus Fe can deposit from SC-i and Al from SC-2 solutions,4 with deposition of noble metals such as Cu from HF-based etch solutions a particularly common problem.56 Metal contamination during aqueous processing has re- cently been the object of intense research,3-" since metal surface contamination can decrease yield in semiconductor manufacturing by causing a variety of electrical problems.3'678 A striking example of the sensitivity to metal contamination is the limit of 1010 metal atom/cm2 that has been widely quoted as the tolerance level for 16 megabyte (MB) dynamic random access memory (DRAM) devices.'2'4 Numerous metal deposition mechanisms have been suggested,6'8'13"5 including precipitation from a supersaturated cleaning solution, incorporation into the silicon dioxide layer during oxide regrowth, and the most problematic, a proposed electroless process whereby metal ion reduction and silicon oxidation occur simultaneously0 In a F-contaming solution Si is a strong reducing agent, since the following half-reaction can occur。
with a standard oxidation potential E' of 1.2 V. 16 The thermodynamics of electroless deposition might then be expected to obey the Nernst equation, so that below a given contaminant level deposition would not occur.6 According to this theory only metals with reduction potentials more noble than hydrogen evolution should deposit. Surprisingly the Nernst equation has failed to quantitatively account for deposition of Fe, Ni, and Zn from dilute HF:H70. 6 Studies of metallic contamination during aqueous pro- cessing are limited in part by the difficulty of monitoring。
上一篇: 芯片制造中的电化学加工技术:挑战与机遇