Abstract
SnO2 fifilms were deposited by atmospheric pressure chemical vapor deposition (APCVD) on glass substrates using tin tetrachloride as the tin precursor, H2O vapor and O3–O2 as oxidizing agents and O3–O2 with HF as the flfluorine dopant source. The deposition temperatures varied from 200 to 350 1C. It is shown that the deposition temperature and the oxidizing agent used are related with the structure, optical transmission percent, and resistivities of the fifilms. Finally, fifilms with good transmission percent between 85% and 90% in the visible spectrum and lower resistivities ranged from 0.1 to 0.02 O cm are obtained. r 2007 Elsevier Ltd. All rights reserved.
1. Introduction Transparent highly conducting oxide (TCO) fifilms are commonly used in many technically signifificant applications, especially for electronic devices such as solar cells [1], flflat panel displays [2], and gas sensors [3]. Metallic oxide thin fifilms, e.g., oxides of indium, tin, and zinc, and in particular indium–tin oxide (ITO) [4], have been fabricated by a variety of deposition techniques such as spray pyrolysis [5], electron beam evaporation [6], magnetron sputtering [7,8] and chemical vapor deposition (CVD) [9,10]. Generally, SnO2 fifilms deposited on glass by CVD are polycrystalline in nature [11]. Electrical properties of SnO2 layers deposited on glass vary depending on the growth technique, choice of tin precursor and substrate, fifilm thickness, and deposition temperature [4,12]. For example, chloride-based precursors are known to produce thin fifilms with resistivities in the range of 0.1–12 Ocm with deposition temperatures from 400 to 500 1C and transmission percent of 90% [13]. In this paper we present a homemade atmospheric pressure chemical vapor deposition (APCVD) system for SnO2 fifilms deposition obtained at very low deposition temperatures, using SnCl4 as precursor, H2O vapor and ozone as oxidizing agents and HF as the flfluorine dopant source. Also, structural, optical, and electrical characterizations of the fifilms deposited under different conditions of growth are presented.
2. Experimental Tin oxide fifilms were deposited on commercial glass substrates in a vertical cold-wall APCVD system, where the susceptor of graffifito was heated by an AC signal (10 Vp, 60 Hz). The reactor was made of quartz and a Eurotherm 2416 temperature controller was used. As tin precursor, it was used tin tetraclorure, H2O vapor and O3 as oxidizing agents and HF as flfluorine dopant source. A schematic diagram of the growth system is illustrated in Fig. 1. The best conditions for the deposit of the samples using H2O were of 1 and 2 slm of N2 (carrier gas), that bubbles the H2O and SnCl4 reagents, respectively. For the case where ozone is used as oxidizing agent, the rate of oxygen flflow was of 0.5 slm, containing 5% of ozone and 1 slm of N2 that bubbles SnCl4, and 2 slm of HF, when the SnO2 fifilms were doped. The SnCl4 vapor pressure was of 2.4 KPa. For all the cases the temperature of the SnCl4 container was maintained at room temperature and the growth temperatures were ranged from 200 to 350 1C.The thickness of the deposited tin oxide fifilms were determined using an ellipsometer Rudolph 439L633P. The X-ray diffraction (XRD) patterns mesurements were done in a hi-res Bruker Axs D8 Discover diffractometer using the monochromatic Cu Kp radiation (l ¼ 1.5418 A˚ ) with the dust technique. The X-ray Bruker Axs D8 source was operated at 40 kV and 40 mA.The optical characterization was carried out by means of transmitance using a spectrophotometer Shimadzu UV-2401 (PC) 120 V ranged from 200 to 1100 nm.The conductance was obtained by means of the four point prove using aluminum contacts.
3. Result and discussions Fig. 2 shows XRD patterns for SnO2 fifilms using H2O (Fig. 2a), O3 (Fig. 2b) and O3 doped with flfluorine (Fig. 2c). All fifilms present tetragonal rutile structure. Their deposition temperatures were varied from 200 to 350 1C with a deposition time of 30 min. In the case where H2O is used as the oxidizing agent (Fig. 2a), it is observed that initially, at 200 1C, the fifilm has a better crystallinity with direction preferred in (2,0,0). Later, the fifilm structure changes to polycrystalline without a preferred direction. This behavior can take place, since, initially an oriented crystalline growth is obtained as a result of a preferential nucleation on the crystalline surface in growth and later, with the increase of the temperature, the adherence of homogenous nuclei is increased from the gaseous phase reducing the oriented growth and increasing the random direction growth. In the case where O3 is used as oxidizing agent (Fig. 2b) it is observed that at low temperatures the structure of the fifilm corresponds to an intermediary Sn3O4 structure, and later, when the temperature is increased at 275 1C and higher, the fifilm turn to be polycrystalline without a preferred direction. Unlike the previous case, this can take place since the deposition mechanism is carried out by an initial nucleation on a bare substrate and by the growth of nuclei due to the surface diffusion of the impinging flflux and, therefore, does not have a growth with preferred orientation. When flfluorine impurities are added to the fifilm (Fig. 2c) an increase of the preferential direction (1,1,0) is observed as it was observed by Saxena et al. [14] where a suitable flfluorine atom concentration corresponds to a greater 31.1 to 56.85 nm.
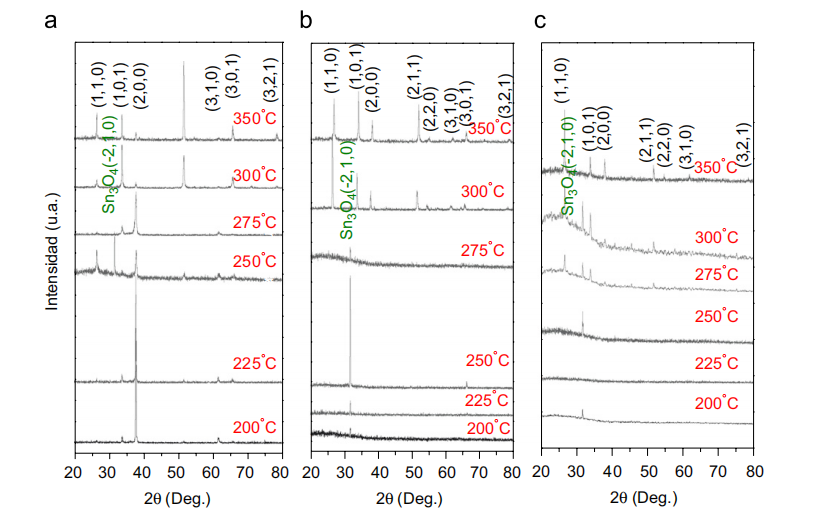
Fig. 2. X-ray diffraction patterns of tin oxide fifilms deposited by APCVD using tin tetrachloride and (a) H2O vapors, (b) ozone, and (c) F–O3. All the samples were obtained to the same deposition temperatures and depositions times. The structures were obtained by an X-ray Bruker Axs D8 source.
下一篇: 超薄单晶硅微结构