HAL is a multi-disciplinary open access archive for the deposit and dissemination of scientific research documents, whether they are published or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers. L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
A bstmet Titanium nitride thin films were deposited by atmospheric chemical vapour deposition in the temperature range 560°C to 660°C from titanium tetrachloride and ammonia in argon carrier gas and studied in terms of nucleation and growth, crystalline orientation and impurities. The films were deposited in a cold wall, atmospheric pressure CVD reactor designed to encourage laminar flow conditions and accommodate a number of different substrates under similar temperature and mass transport conditions. Characterisation of the films using scanning electron and atomic force microscopy showed an increase in nucleation density and decrease in surface roughness with temperature. Giancingangle X-ray diffraction determined the crystallinity and orientation of the films with respect to the substrate and deposition temperature. Films deposited on Si,N, showed preferred orientation whereas those on glass showed random orientation. Energy dispersive spectroscopy calibrated by Rutherford backscattering spectroscopy indicated that the amount of chlorine and oxygen contamination decreased with increasing temperature. RBS also determined the stoichiometry of the titanium nitride films. Resistivity and optical studies were also carried out on titanium nitride thin films on glass to evaluate their suitability as heat mirrors.
Titanium nitride is a gold coloured solid that is almost as hard as diamond (9-10 Mohs [I]), has a greater electrical conductivity than titanium metal (aTi,=4608kScm-', 0,=2381 kScmS' [I]), and is highly reflective in the infrared region of the electromagnetic spectrum while transmitting in the optical region. These properties have lead to research into uses for machine tool coatings, aluminium diffusion barriers in integrated circuits and heat mirror applications (a thin layer of TiN appearing green in transmission). Heat mirrors are of environmental importance since they reduce space heating in cold climates or air conditioning requirements in equatorial climates thus reducing energy consumption. Titanium nitride is deposited by both physical and chemical vapour deposition techniques. Chemical vapour deposition from titanium tetrachloride and a nitrogen and hydrogen mixture is thermodynamically favourable at 850°C but temperatures above 1000°C and as high as 2000°C 12-51 are generally used for durable machine tool coatings since this produces high quality very hard wearing TiN. However these conditions are too harsh to be applied to integrated circuits and limits coating applications on glass. Physical vapour deposition from Ti targets and nitrogen in argon produces high purity TIN on temperature sensitive substrates, such as those used in IC technology, but unsuitable for large area deposition [6]. TiN from metal organic precursors such as tetrakis-dimethylamide titanium and ammonia brings the deposition temperature by CVD down to approximately 200°C to 300°C but these films suffer carbon, hydrocarbon, carbide and oxygen impurities [7-121. Other more reactive species are being considered such as titanium azides (Cp,Ti(N,),) [I31 and FTi[N(SiMe3)J3 [14]. Kurtz and Gordon looked into the atmospheric pressure chemical vapour deposition of titanium nitride from titanium tetrachloride and ammonia (450°C to 750°C) [4] which react at room temperature to form a complex. This then decomposes with further heating to form TiNCl at 350°C and TiN above 450°C as researched in the early 1950s by Fowles and Pollard, Antler and Laubengayer [15,16]. At lower temperatures (450°C to 600°C) the TiN thin films suffer some chlorine and oxygen contamination and growth rates are slow compared to OMCVD routes. The majority of research has been into low pressure CVD of this chemistry 1171 so Kurtz and Gordon's paper will becited most in this present research. Although this process would be unsuitable for IC technology it could be applied to heat mirror coatings.
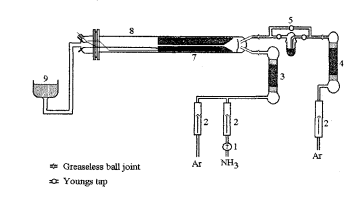
figme 1: Atmospheric pressure CVD cold wall reactor. 1. flowstat, 2. gap flow meter. 3. NH,/Ar drying column, 4. Ar drying column. 5. bubbler, 6. baffle disk, 7. graphite blocks containing heater cartridge and substrates. 8. silica tube, 9. outlet containing HCI scrubber