Most processing of the III nitrides is currently done by dry plasma etching.1,2 There are several disadvantages to dry etching, including the generation of ion-induced damage3 and diffificulty in obtaining smooth etched sidewalls, which are required for lasers. The typical root-mean-square ~ rms! roughness of sidewalls produced by dry etching is on the order of 50 nm,4,5 although recently surfaces with an rms roughness as low as 4–6 nm have been reported.6 Photoenhanced electrochemical ~ PEC! wet etching has also been demonstrated for etching of gallium nitride ~ GaN! . 7–10 PEC etching has the advantage of relatively low equipment cost and low surface damage, but a method for producing smooth vertical sidewalls has not yet been found. Cleaved facets for GaN have also been reported, with rms roughnesses varying between 16 nm for GaN grown on sapphire substrates11 and 0.3 nm for GaN grown on spinel substrates.12
While KOH-based solutions have been found to etch AlN and InAlN, no acid or base solution has previously been identifified that is able to etch high-quality GaN.13 In this letter, we have used ethylene glycol, instead of water, as a solvent for KOH and NaOH so that we are able to employ temperatures between 90 and 180 °C. These temperatures exceed the boiling point of water and are considerably higher than the temperatures used in previous references.13 By so doing, we have developed a two-step process that etches crystallographic surfaces into III nitrides. Our samples are 2- m m-thick n-type GaN epilayers grown on c-plane sapphire by metal-organic chemical vapor deposition ~ MOCVD! , and the fifilms have an x-ray diffraction rocking curve full width at half maximum of ; 800 arcsec.14
Molten KOH and hot phosphoric acid ~ H3PO4! have previously been shown to etch pits at defect sites in the c plane of GaN.15,16 Kozawa et al. reported that the facets of the pits correspond to the $ 303¯2% face of GaN.16 We have observed the formation of etch pits with facets that correspond to various GaN crystal faces by etching in H3PO4 above 160 °C, in molten KOH above 180 °C, in KOH dissolved in ethylene glycol above 135 °C, and in NaOH dissolved in ethylene glycol at 180 °C. All of the hexagonal etch pits share a common base, i.e., the ^ 112¯0& direction, but intersect the c planeat a wide variety of angles. This is because the faces are actually produced by two or more competing etch planes, as can be seen in the high-resolution fifield-effect scanning electron microscope ~ FESEM! image in Fig. 1. The etchant temperatures are monitored using a thermocouple, and are accurate to within 5 °C. The etch pit density is approximately 23 106 cm 2 2 in H3PO4 and 63 107 cm2 2 in hydroxidecontaining etchants.
The fifirst of the two etching steps in the crystallographic etching process is used to establish the etching depth, and it can be performed by several common processing methods. For our fifirst step we have used several different processing methods, including reactive ion etching in a chlorine-based plasma, PEC etching in a KOH solution, and cleaving. The second step is done by immersion in a chemical that is able to crystallographically etch GaN. This etching step can produce smooth crystallographic surfaces, and the specifific etching planes can be chosen by varying the orientation of the fifirst step, the chemical agents, and the temperature. The etch rates and crystal planes observed for all chemicals used in this work are summarized in Table I. The etching planes listed in this table are those that appear during the etch. Because the c plane $ 0001% is impervious to all of these chemicals except at defect sites where etch pits occur, it is also an etch plane, with a negligibly small etch rate.
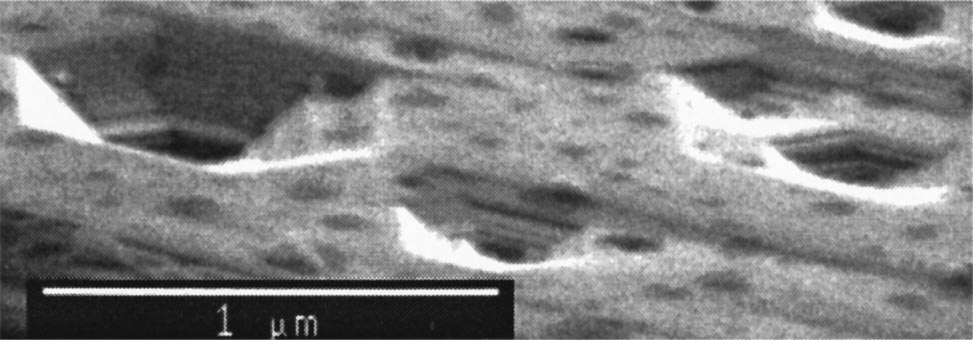
Fig1
The activation energies for etching in these various solutions is 0.9 eV, or 21 kcal/mol, as inferred from the Arrhenius plots in Figs. 3 and 4. Note that this is equal to the calculated heat of formation of GaN, 0.90 eV.17 The activation energy indicates that the etch is reaction-rate limited. If the etching were diffusion limited, an activation energy in the 1–6 kcal/mol range would be expected.18
It is interesting to note that the etch rate of KOH dissolved in ethylene glycol is higher than the etch rate of molten KOH at the same temperature. In fact, the etch rate as a function of concentration peaks at a value of 40% KOH by weight in ethylene glycol, as can be seen in Fig. 5. We believe that this is due to high solubility of the etch products in ethylene glycol.
Because the c plane is impervious to all of the chemicals used in this study, no etch mask is required for the crystallographic etching step—the c plane itself acts as a mask. An etch mask may be necessary, however, if long etching times are used, to prevent the development of etch pits at defect sites. For this purpose we have successfully used titanium masks after annealing at 900 °C for 30 s.
上一篇: 碳化硅器件加工过程化学浓度控制
下一篇: InGaAs薄膜波导