Polished silicon wafers are prepared through various mechanical and chemical processes. First, the silicon single-crystal ingot is sliced into circular disks (wafers) by slicing followed by a flattening process called lapping that involves scrubbing the wafers using an abrasive slurry.1 The mechanical damage induced during the previous shaping processes is removed by etching which is the focus of this paper. Etching is followed by various unit operations such as polishing and cleaning before it is ready for device fabrication.
The actual reaction mechanism is quite complicated and involves many elementary reactions. Hydrogen and different oxides of nitrogen can evolve. Many rate equations for the dissolution of silicon wafer under different conditions have been proposed.5-7
Sometimes, the identification of the rate-controlling step in a heterogeneous process like this one (mass-transfer vs. reaction) becomes more critical than the knowledge of the actual chemistry in the design of an etcher, because a reaction-controlled etching requires a different design from a mass-transfer controlled etching to produce uniformly etched silicon wafers.
Robbins and Schwartz published a series of papers on acid etching of silicon from 1958 to 1976.8,10-12 They essentially established that for low HF and high HNO3 concentrations, the etching process is greatly influenced by diffusion. Even for the low nitric acid concentrations when the autocatalytic oxidation-reduction reaction influences the etching rate, mass-transfer effects are significant.10,12 Their claim is supported by observations of Bogenschütz et al. and Klein and D’Stefan among many others.9,13 Bogenschütz et al. showed that “activation energy” for mass-transfer controlled etching is comparable to the activation energy for viscosity.9 Klein and D’Stefan observed a change in the etching rate with change in the mixing rate.13 They observed a decrease in the dependence of the etching rate on the mixing rate with the increase in the HF concentration. This dependence was monotonic and linear. This means that the kinetic effects influence the etching process along with masstransfer effects as the concentration of HF increases. Erk and Vandamme describe a process for chemical etching of silicon wafers where nitrogen bubbling is used for uniform mass-transfer effects.14
Phenomenological Model: Two-Phase System
The etching process itself typically involves the following steps (Fig. 1a): (i) transport of the reactants from the bulk solution to the wafer surface, (ii) effective reaction(s) on the wafer surface, and (iii) transport of products from the wafer surface to the bulk solution.
Reactants pass through a stagnant liquid film, which offers a finite resistance for mass-transfer before reactants reach the surface of the wafer. Products also pass through the mass-transfer film (henceforward, the term film refers to mass-transfer film unless specified otherwise). Hence, the reaction and physical transport of reagents occur as steps in series. The finite rate of chemical kinetics provides a finite kinetic resistance that acts in series with the masstransfer resistance (Fig. 1a and b). When the mass-transfer and kinetic (reactive) resistances are comparable in magnitude, both kinetics and mass-transfer affect the rate of etching. However, when the difference in the kinetic and mass-transfer resistances is appreciable, the step with higher resistance controls the rate of acid etching. The phenomenological model shown in Fig. 1a and b is applicable to any typical solid-liquid system.
Phenomenological Model: Three-Phase System
Acid etching of silicon wafers is greatly influenced by gaseous products. In the phenomenological model presented in the previous section, the effect of gaseous products on the etching rate was not addressed. Macromodeling of etching must incorporate (i) transport of the reactants in the liquid phase from the bulk solution to the wafer surface, (ii) effective reaction(s) on the wafer surface to produce products in both liquid and gas phase, (iii) detachment of gaseous products (bubbles) from the silicon surface, and (iv) transport of the gaseous products (bubbles) to the bulk phase.

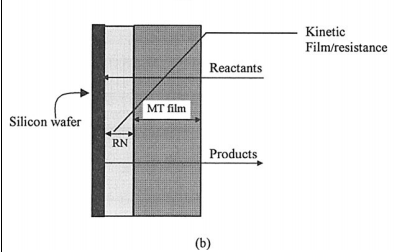
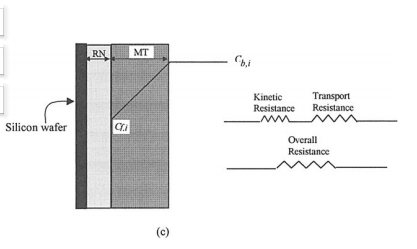
Fig1
Phenomenological Model: Three-Phase System
Acid etching of silicon wafers is greatly influenced by gaseous products. In the phenomenological model presented in the previous section, the effect of gaseous products on the etching rate was not addressed. Macromodeling of etching must incorporate (i) transport of the reactants in the liquid phase from the bulk solution to the wafer surface, (ii) effective reaction(s) on the wafer surface to produce products in both liquid and gas phase, (iii) detachment of gaseous products (bubbles) from the silicon surface, and (iv) transport of the gaseous products (bubbles) to the bulk phase.
Experimental
The above phenomenological model was proposed using classical chemical engineering fundamentals to explain experimental data collected under various conditions. In this study we discuss the nature of these experiments and qualitatively analyze collected data using the proposed phenomenological model.
上一篇: 碳化硅和二氧化硅之间稳定性的刻蚀选择性
下一篇: 太阳能电池制造中化学浓度的先进工艺控制